Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli
- PMID: 16251436
- PMCID: PMC6725570
- DOI: 10.1523/JNEUROSCI.2019-05.2005
Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli
Abstract
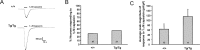
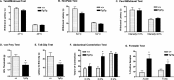
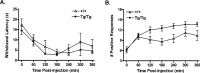
Molecular and behavioral evidence suggests that acid-sensing ion channels (ASICs) contribute to pain processing, but an understanding of their precise role remains elusive. Existing ASIC knock-out mouse experiments are complicated by the heteromultimerization of ASIC subunits. Therefore, we have generated transgenic mice that express a dominant-negative form of the ASIC3 subunit that inactivates all native neuronal ASIC-like currents by oligomerization. Using whole-cell patch-clamp recordings, we examined the response properties of acutely isolated dorsal root ganglion neurons to protons (pH 5.0). We found that whereas 33% of the proton-responsive neurons from wild-type mice exhibited an ASIC-like transient response, none of the neurons from the transgenic mice exhibited a transient inward current. Capsaicin-evoked responses mediated by the TRPV1 receptor were unaltered in transgenic mice. Adult male wild-type and transgenic mice were subjected to a battery of behavioral nociceptive assays, including tests of thermal, mechanical, chemical/inflammatory, and muscle pain. The two genotypes were equally sensitive to thermal pain and to thermal hypersensitivity after inflammation. Compared with wild types, however, transgenic mice were more sensitive to a number of modalities, including mechanical pain (von Frey test, tail-clip test), chemical/inflammatory pain (formalin test, 0.6% acetic acid writhing test), mechanical hypersensitivity after zymosan inflammation, and mechanical hypersensitivity after intramuscular injection of hypotonic saline. These data reinforce the hypothesis that ASICs are involved in both mechanical and inflammatory pain, although the increased sensitivity of transgenic mice renders it unlikely that they are direct transducers of nociceptive stimuli.
Figures
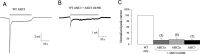


References
-
- Adams CM, Snyder PM, Welsh MJ (1999) Paradoxical stimulation of a DEG/ENaC channel by amiloride. J Biol Chem 274: 15500-15504. - PubMed
-
- Anzai N, Deval E, Schaefer L, Friend V, Lazdunski M, Lingueglia E (2002) The multivalent PDZ domain-containing protein CIPP is a partner of acid-sensing ion channel 3 in sensory neurons. J Biol Chem 277: 16655-16661. - PubMed
-
- Babinski K, Le KT, Seguela P (1999) Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem 72: 51-57. - PubMed
-
- Baumann TK, Chaudhary P, Martenson ME (2004) Background potassium channel block and TRPV1 activation contribute to proton depolarization of sensory neurons from humans with neuropathic pain. Eur J Neurosci 19: 1343-1351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
