Neural correlates of reach errors
- PMID: 16251440
- PMCID: PMC1479774
- DOI: 10.1523/JNEUROSCI.1874-05.2005
Neural correlates of reach errors
Abstract
Reach errors may be broadly classified into errors arising from unpredictable changes in target location, called target errors, and errors arising from miscalibration of internal models (e.g., when prisms alter visual feedback or a force field alters limb dynamics), called execution errors. Execution errors may be caused by miscalibration of dynamics (e.g., when a force field alters limb dynamics) or by miscalibration of kinematics (e.g., when prisms alter visual feedback). Although all types of errors lead to similar on-line corrections, we found that the motor system showed strong trial-by-trial adaptation in response to random execution errors but not in response to random target errors. We used functional magnetic resonance imaging and a compatible robot to study brain regions involved in processing each kind of error. Both kinematic and dynamic execution errors activated regions along the central and the postcentral sulci and in lobules V, VI, and VIII of the cerebellum, making these areas possible sites of plastic changes in internal models for reaching. Only activity related to kinematic errors extended into parietal area 5. These results are inconsistent with the idea that kinematics and dynamics of reaching are computed in separate neural entities. In contrast, only target errors caused increased activity in the striatum and the posterior superior parietal lobule. The cerebellum and motor cortex were as strongly activated as with execution errors. These findings indicate a neural and behavioral dissociation between errors that lead to switching of behavioral goals and errors that lead to adaptation of internal models of limb dynamics and kinematics.
Figures
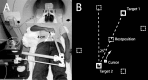
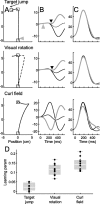
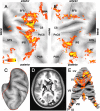



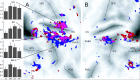

References
-
- Atkeson CG (1989) Learning arm kinematics and dynamics. Annu Rev Neurosci 12: 157-183. - PubMed
-
- Baizer JS, Kralj-Hans I, Glickstein M (1999) Cerebellar lesions and prism adaptation in macaque monkeys. J Neurophysiol 81: 1960-1965. - PubMed
-
- Battaglia-Mayer A, Ferraina S, Mitsuda T, Marconi B, Genovesio A, Onorati P, Lacquaniti F, Caminiti R (2000) Early coding of reaching in the parietooccipital cortex. J Neurophysiol 83: 2374-2391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
