Vascular development of the brain requires beta8 integrin expression in the neuroepithelium
- PMID: 16251442
- PMCID: PMC2849654
- DOI: 10.1523/JNEUROSCI.3467-05.2005
Vascular development of the brain requires beta8 integrin expression in the neuroepithelium
Abstract
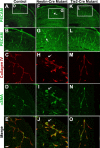
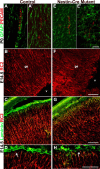
We showed previously that loss of the integrin beta8 subunit, which forms alphavbeta8 heterodimers, results in abnormal vascular development in the yolk sac, placenta, and brain. Animals lacking the integrin beta8 (itgbeta8) gene die either at midgestation, because of insufficient vascularization of the placenta and yolk sac, or shortly after birth with severe intracerebral hemorrhage. To specifically focus on the role of integrins containing the beta8 subunit in the brain, and to avoid early lethalities, we used a targeted deletion strategy to delete itgbeta8 only from cell types within the brain. Ablating itgbeta8 from vascular endothelial cells or from migrating neurons did not result in cerebral hemorrhage. Targeted deletion of itgbeta8 from the neuroepithelium, however, resulted in bilateral hemorrhage at postnatal day 0, although the phenotype was less severe than in itgbeta8-null animals. Newborn mice lacking itgbeta8 from the neuroepithelium had hemorrhages in the cortex, ganglionic eminence, and thalamus, as well as abnormal vascular morphogenesis, and disorganized glia. Interestingly, adult mice lacking itgbeta8 from cells derived from the neuroepithelium did not show signs of hemorrhage. We propose that defective association between vascular endothelial cells and glia lacking itgbeta8 is responsible for the leaky vasculature seen during development but that an unidentified compensatory mechanism repairs the vasculature after birth.
Figures




References
-
- Abbott NJ, Romero IA (1996) Transporting therapeutics across the blood-brain barrier. Mol Med Today 2: 106-113. - PubMed
-
- Bader BL, Rayburn H, Crowley D, Hynes RO (1998) Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell 95: 507-519. - PubMed
-
- Breier G, Albrecht U, Sterrer S, Risau W (1992) Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 114: 521-532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
