Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion
- PMID: 16251452
- PMCID: PMC6725568
- DOI: 10.1523/JNEUROSCI.2652-05.2005
Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion
Abstract
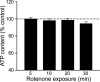
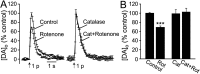
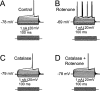
Mitochondrial dysfunction is a potential causal factor in Parkinson's disease. We show here that acute exposure to the mitochondrial complex I inhibitor rotenone (30-100 nM; 30 min) causes concentration-dependent suppression of single-pulse evoked dopamine (DA) release monitored in real time with carbon-fiber microelectrodes in guinea pig striatal slices, with no effect on DA content. Suppression of DA release was prevented by the sulfonylurea glibenclamide, implicating ATP-sensitive K+ (KATP) channels; however, tissue ATP was unaltered. Because KATP channels can be activated by hydrogen peroxide (H2O2), as well as by low ATP, we examined the involvement of rotenone-enhanced H2O2 generation. Confirming an essential role for H2O2, the inhibition of DA release by rotenone was prevented by catalase, a peroxide-scavenging enzyme. Striatal H2O2 generation during rotenone exposure was examined in individual medium spiny neurons using fluorescence imaging with dichlorofluorescein (DCF). An increase in intracellular H2O2 levels followed a similar time course to that of DA release suppression and was accompanied by cell membrane depolarization, decreased input resistance, and increased excitability. Extracellular catalase markedly attenuated the increase in DCF fluorescence and prevented rotenone-induced effects on membrane properties; membrane changes were also largely prevented by flufenamic acid, a blocker of transient receptor potential (TRP) channels. Thus, partial mitochondrial inhibition can cause functional DA denervation via H2O2 and KATP channels, without DA or ATP depletion. Furthermore, amplified H2O2 levels and TRP channel activation in striatal spiny neurons indicate potential sources of damage in these cells. Overall, these novel factors could contribute to parkinsonian motor deficits and neuronal degeneration caused by mitochondrial dysfunction.
Figures






Similar articles
-
Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels.J Neurosci. 2005 Apr 27;25(17):4222-31. doi: 10.1523/JNEUROSCI.4701-04.2005. J Neurosci. 2005. PMID: 15858048 Free PMC article.
-
Activation of ATP-sensitive K+ (K(ATP)) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release.Proc Natl Acad Sci U S A. 2003 Sep 30;100(20):11729-34. doi: 10.1073/pnas.1834314100. Epub 2003 Sep 17. Proc Natl Acad Sci U S A. 2003. PMID: 13679582 Free PMC article.
-
Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling.J Neurosci. 2009 Jul 15;29(28):9002-10. doi: 10.1523/JNEUROSCI.1706-09.2009. J Neurosci. 2009. PMID: 19605638 Free PMC article.
-
Classification of H₂O₂as a neuromodulator that regulates striatal dopamine release on a subsecond time scale.ACS Chem Neurosci. 2012 Dec 19;3(12):991-1001. doi: 10.1021/cn300130b. Epub 2012 Nov 8. ACS Chem Neurosci. 2012. PMID: 23259034 Free PMC article. Review.
-
H2O2 signaling in the nigrostriatal dopamine pathway via ATP-sensitive potassium channels: issues and answers.Antioxid Redox Signal. 2007 Feb;9(2):219-31. doi: 10.1089/ars.2007.9.219. Antioxid Redox Signal. 2007. PMID: 17115944 Review.
Cited by
-
Both stereoselective (R)- and (S)-1-Methyl-1,2,3,4-tetrahydroisoquinoline enantiomers protect striatal terminals against rotenone-induced suppression of dopamine release.Neurotox Res. 2011 Aug;20(2):134-49. doi: 10.1007/s12640-010-9228-5. Epub 2010 Nov 11. Neurotox Res. 2011. PMID: 21069490 Free PMC article.
-
Ether-à-go-go 1 (Eag1) potassium channel expression in dopaminergic neurons of basal ganglia is modulated by 6-hydroxydopamine lesion.Neurotox Res. 2012 Apr;21(3):317-33. doi: 10.1007/s12640-011-9286-3. Epub 2011 Nov 3. Neurotox Res. 2012. PMID: 22048886
-
Reactive oxygen species increase neuronal excitability via activation of nonspecific cation channel in rat medullary dorsal horn neurons.Korean J Physiol Pharmacol. 2017 Jul;21(4):371-376. doi: 10.4196/kjpp.2017.21.4.371. Epub 2017 Jun 26. Korean J Physiol Pharmacol. 2017. PMID: 28706450 Free PMC article.
-
Selective and Mechanically Robust Sensors for Electrochemical Measurements of Real-Time Hydrogen Peroxide Dynamics in Vivo.Anal Chem. 2018 Jan 2;90(1):888-895. doi: 10.1021/acs.analchem.7b03770. Epub 2017 Dec 15. Anal Chem. 2018. PMID: 29191006 Free PMC article.
-
Ketones prevent synaptic dysfunction induced by mitochondrial respiratory complex inhibitors.J Neurochem. 2010 Jul;114(1):130-41. doi: 10.1111/j.1471-4159.2010.06728.x. Epub 2010 Apr 2. J Neurochem. 2010. PMID: 20374433 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
