Successful receptor-mediated radiation therapy of xenografted human midgut carcinoid tumour
- PMID: 16251870
- PMCID: PMC2361494
- DOI: 10.1038/sj.bjc.6602845
Successful receptor-mediated radiation therapy of xenografted human midgut carcinoid tumour
Abstract
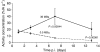
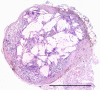
Somatostatin receptor (sstr)-mediated radiation therapy is a new therapeutic modality for neuroendocrine (NE) tumours. High expression of sstr in NE tumours leads to tumour-specific uptake of radiolabelled somatostatin analogues and high absorbed doses. In this study, we present the first optimised radiation therapy via sstr using [(177)Lu-DOTA(0)-Tyr(3)]-octreotate given to nude mice xenografted with the human midgut carcinoid GOT1. The tumours in 22 out of 23 animals given therapeutic amounts showed dose-dependent, rapid complete remission. The diagnostic amount (0.5 MBq [(177)Lu-DOTA(0)-Tyr(3)]-octreotate) did not influence tumour growth and was rapidly excreted. In contrast, the therapeutic amount (30 MBq [(177)Lu-DOTA(0)-Tyr(3)]-octreotate) induced rapid tumour regression and entrapment of (177)Lu so that the activity concentration of (177)Lu remained high, 7 and 13 days after injection. The entrapment phenomenon increased the absorbed dose to tumours from 1.6 to 4.0 Gy MBq(-1) and the tumours in animals treated with 30 MBq received 120 Gy. Therapeutic amounts of [(177)Lu-DOTA(0)-Tyr(3)]-octreotate rapidly induced apoptosis and gradual development of fibrosis in grafted tumours. In conclusion, human midgut carcinoid xenografts can be cured by receptor-mediated radiation therapy by optimising the uptake of radioligand and taking advantage of the favourable change in biokinetics induced by entrapment of radionuclide in the tumours.
Figures
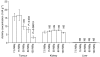



Similar articles
-
Comparison of [177Lu-DOTA0,Tyr3]-octreotate and [177Lu-DOTA0,Tyr3]-octreotide for receptor-mediated radiation therapy of the xenografted human midgut carcinoid tumor GOT1.Cancer Biother Radiopharm. 2008 Feb;23(1):114-20. doi: 10.1089/cbr.2007.0421. Cancer Biother Radiopharm. 2008. PMID: 18298335
-
GOT1 xenografted to nude mice: a unique model for in vivo studies on SSTR-mediated radiation therapy of carcinoid tumors.Ann N Y Acad Sci. 2004 Apr;1014:275-9. doi: 10.1196/annals.1294.031. Ann N Y Acad Sci. 2004. PMID: 15153445
-
Effects of treatment with (177)Lu-DOTA-Tyr(3)-octreotate on uptake of subsequent injection in carcinoid-bearing nude mice.Cancer Biother Radiopharm. 2007 Oct;22(5):644-53. doi: 10.1089/cbr.2007.333. Cancer Biother Radiopharm. 2007. PMID: 17979567
-
Treatment of advanced neuroendocrine tumours with radiolabelled somatostatin analogues.Endocr Relat Cancer. 2005 Dec;12(4):683-99. doi: 10.1677/erc.1.01116. Endocr Relat Cancer. 2005. PMID: 16322317 Review.
-
Peptide Receptor Radionuclide Therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours.Acta Oncol. 2007;46(6):723-34. doi: 10.1080/02841860701441848. Acta Oncol. 2007. PMID: 17653893 Review.
Cited by
-
The neuroendocrine phenotype, genomic profile and therapeutic sensitivity of GEPNET cell lines.Endocr Relat Cancer. 2018 Mar;25(3):367-380. doi: 10.1530/ERC-17-0445. Endocr Relat Cancer. 2018. PMID: 29444910 Free PMC article.
-
Neuroblastoma xenograft models demonstrate the therapeutic potential of 177Lu-octreotate.BMC Cancer. 2021 Aug 25;21(1):950. doi: 10.1186/s12885-021-08551-8. BMC Cancer. 2021. PMID: 34433438 Free PMC article.
-
Translational research in neuroendocrine tumors: pitfalls and opportunities.Oncogene. 2017 Apr 6;36(14):1899-1907. doi: 10.1038/onc.2016.316. Epub 2016 Sep 19. Oncogene. 2017. PMID: 27641330 Review.
-
Tumour size measurement in a mouse model using high resolution MRI.BMC Med Imaging. 2012 May 30;12:12. doi: 10.1186/1471-2342-12-12. BMC Med Imaging. 2012. PMID: 22647088 Free PMC article.
-
Stroma targeting nuclear imaging and radiopharmaceuticals.Int J Mol Imaging. 2012;2012:817682. doi: 10.1155/2012/817682. Epub 2012 May 21. Int J Mol Imaging. 2012. PMID: 22685650 Free PMC article.
References
-
- Ahlman H, Nilsson O, Olausson M (2004) Interventional treatment of the carcinoid syndrome. Neuroendocrinology 80(Suppl 1): 67–73 - PubMed
-
- Ahlman H, Wängberg B, Jansson S, Friman S, Olausson M, Tylen U, Nilsson O (2000) Interventional treatment of gastrointestinal neuroendocrine tumours. Digestion 62(Suppl 1): 59–68 - PubMed
-
- Ahlman H, Wängberg B, Tisell LE, Nilsson O, Fjälling M, Forssell-Aronsson E (1994) Clinical efficacy of octreotide scintigraphy in patients with midgut carcinoid tumours and evaluation of intraoperative scintillation detection. Br J Surg 81: 1144–1149 - PubMed
-
- Bernhardt P, Ahlman H, Forssell-Aronsson E (2003a) Model of metastatic growth valuable for radionuclide therapy. Med Phys 30: 3227–3232 - PubMed
-
- Bernhardt P, Ahlman H, Nilsson O, Benjegård SA, Forssell-Aronsson E (2003b) Evaluation of (111)In labeled somatostatin analogs for targeted therapy of somatostatin receptor positive tumors. Cancer Biother Radiopharm 18: 249–252 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

