Critical role of novel Thr-219 autophosphorylation for the cellular function of PKCtheta in T lymphocytes
- PMID: 16252004
- PMCID: PMC1283955
- DOI: 10.1038/sj.emboj.7600856
Critical role of novel Thr-219 autophosphorylation for the cellular function of PKCtheta in T lymphocytes
Abstract
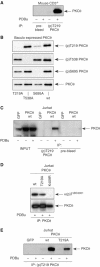
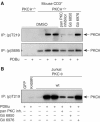
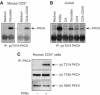
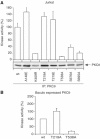
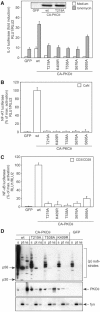
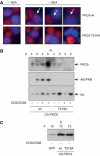
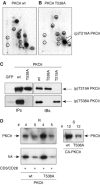
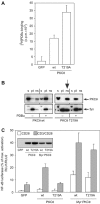
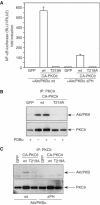
Phosphopeptide mapping identified a major autophosphorylation site, phospho (p)Thr-219, between the tandem C1 domains of the regulatory fragment in protein kinase C (PKC)theta. Confirmation of this identification was derived using (p)Thr-219 antisera that reacted with endogenous PKCtheta in primary CD3+ T cells after stimulation with phorbol ester, anti-CD3 or vanadate. The T219A mutation abrogated the capacity of PKCtheta to mediate NF-kappaB, NF-AT and interleukin-2 promoter transactivation, and reduced PKCtheta's ability in Jurkat T cells to phosphorylate endogenous cellular substrates. In particular, the T219A mutation impaired crosstalk of PKCtheta with Akt/PKBalpha in NF-kappaB activation. Yet, this novel (p)Thr-219 site did not affect catalytic activity or second-messenger lipid-binding activity in vitro. Instead, the T219A mutation prevented proper recruitment of PKCtheta in activated T cells. The PKCthetaT219A mutant defects were largely rescued by addition of a myristoylation signal to force its proper membrane localization. We conclude that autophosphorylation of PKCtheta at Thr-219 plays an important role in the correct targeting and cellular function of PKCtheta upon antigen receptor ligation.
Figures

Similar articles
-
AKT1/PKBalpha is recruited to lipid rafts and activated downstream of PKC isotypes in CD3-induced T cell signaling.FEBS Lett. 2003 Apr 24;541(1-3):155-62. doi: 10.1016/s0014-5793(03)00287-4. FEBS Lett. 2003. PMID: 12706837
-
Protein kinase ctheta cooperates with calcineurin to induce Fas ligand expression during activation-induced T cell death.J Immunol. 1999 Dec 1;163(11):5813-9. J Immunol. 1999. PMID: 10570264
-
Translocation of PKC[theta] in T cells is mediated by a nonconventional, PI3-K- and Vav-dependent pathway, but does not absolutely require phospholipase C.J Cell Biol. 2002 Apr 15;157(2):253-63. doi: 10.1083/jcb.200201097. Epub 2002 Apr 15. J Cell Biol. 2002. PMID: 11956228 Free PMC article.
-
Determining the destiny of NF-kappa B after TCR ligation: it's CARMA1.Mol Interv. 2002 Oct;2(6):356-60, 338. doi: 10.1124/mi.2.6.356. Mol Interv. 2002. PMID: 14993411 Review.
-
Protein kinase C theta (PKCtheta): a key player in T cell life and death.Pharmacol Res. 2007 Jun;55(6):537-44. doi: 10.1016/j.phrs.2007.04.009. Epub 2007 May 1. Pharmacol Res. 2007. PMID: 17544292 Free PMC article. Review.
Cited by
-
In and out of the bull's eye: protein kinase Cs in the immunological synapse.Trends Immunol. 2013 May;34(5):234-42. doi: 10.1016/j.it.2013.01.002. Epub 2013 Feb 19. Trends Immunol. 2013. PMID: 23428395 Free PMC article. Review.
-
TCR-induced sumoylation of the kinase PKC-θ controls T cell synapse organization and T cell activation.Nat Immunol. 2015 Nov;16(11):1195-203. doi: 10.1038/ni.3259. Epub 2015 Sep 21. Nat Immunol. 2015. PMID: 26390157
-
PKN1 Exerts Neurodegenerative Effects in an In Vitro Model of Cerebellar Hypoxic-Ischemic Encephalopathy via Inhibition of AKT/GSK3β Signaling.Biomolecules. 2023 Oct 31;13(11):1599. doi: 10.3390/biom13111599. Biomolecules. 2023. PMID: 38002281 Free PMC article.
-
The Novel PKCθ from Benchtop to Clinic.J Immunol Res. 2015;2015:348798. doi: 10.1155/2015/348798. Epub 2015 May 19. J Immunol Res. 2015. PMID: 26090489 Free PMC article. Review.
-
Ceramide inhibits PKCθ by regulating its phosphorylation and translocation to lipid rafts in Jurkat cells.Immunol Res. 2016 Aug;64(4):869-86. doi: 10.1007/s12026-016-8787-9. Immunol Res. 2016. PMID: 26798039
References
-
- Baier G (2003) The PKC gene module: molecular biosystematics to resolve its T cell functions. Immunol Rev 192: 64–79 - PubMed
-
- Bauer B, Baier G (2002) PKC and AKT/PKB in CD4+ T-lymphocytes: new partners in TCR/CD28 signal integration. Mol Immunol 38: 1087–1099 - PubMed
-
- Bauer B, Jenny M, Fresser F, Uberall F, Baier G (2003) AKT1/PKBα is recruited to lipid rafts and activated downstream of PKC isotypes in CD3-induced T cell signaling. FEBS Lett 541: 155–162 - PubMed
-
- Bauer B, Krumbock N, Fresser F, Hochholdinger F, Spitaler M, Simm A, Uberall F, Schraven B, Baier G (2001) Complex formation and cooperation of PKCθ and Akt1/PKBα in the NF-κB transactivation cascade in Jurkat T cells. J Biol Chem 15: 15 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

