Ligand-induced transrepression by VDR through association of WSTF with acetylated histones
- PMID: 16252006
- PMCID: PMC1283952
- DOI: 10.1038/sj.emboj.7600853
Ligand-induced transrepression by VDR through association of WSTF with acetylated histones
Retraction in
-
Retraction: 'Ligand-induced transrepression by VDR through association of WSTF with acetylated histones'.EMBO J. 2014 Dec 1;33(23):2881. doi: 10.15252/embj.201470110. EMBO J. 2014. PMID: 25452584 Free PMC article. No abstract available.
Abstract
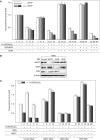
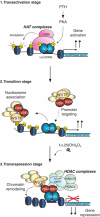
We have previously shown that the novel ATP-dependent chromatin-remodeling complex WINAC is required for the ligand-bound vitamin D receptor (VDR)-mediated transrepression of the 25(OH)D3 1alpha-hydroxylase (1alpha(OH)ase) gene. However, the molecular basis for VDR promoter association, which does not involve its binding to specific DNA sequences, remains unclear. To address this issue, we investigated the function of WSTF in terms of the association between WINAC and chromatin for ligand-induced transrepression by VDR. Results of in vitro experiments using chromatin templates showed that the association of unliganded VDR with the promoter required physical interactions between WSTF and both VDR and acetylated histones prior to VDR association with chromatin. The acetylated histone-interacting region of WSTF was mapped to the bromodomain, and a WSTF mutant lacking the bromodomain served as a dominant-negative mutant in terms of ligand-induced transrepression of the 1alpha(OH)ase gene. Thus, our findings indicate that WINAC associates with chromatin through a physical interaction between the WSTF bromodomain and acetylated his tones, which appears to be indispensable for VDR/promoter association for ligand-induced transrepression of 1alpha(OH)ase gene expression.
Figures





References
-
- Agalioti T, Lomvardas S, Parekh B, Yie J, Maniatis T, Thanos D (2000) Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell 103: 667–678 - PubMed
-
- Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM (1999) Structure and ligand of a histone acetyltransferase bromodomain. Nature 399: 491–496 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

