Clinical and molecular findings in osteoporosis-pseudoglioma syndrome
- PMID: 16252235
- PMCID: PMC1271384
- DOI: 10.1086/497706
Clinical and molecular findings in osteoporosis-pseudoglioma syndrome
Abstract
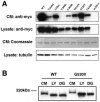
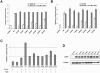
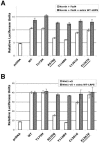
Mutations in the low-density lipoprotein receptor-related protein 5 gene (LRP5) cause autosomal recessive osteoporosis-pseudoglioma syndrome (OPPG). We sequenced the coding exons of LRP5 in 37 probands suspected of having OPPG on the basis of the co-occurrence of severe congenital or childhood-onset visual impairment with bone fragility or osteoporosis recognized by young adulthood. We found two putative mutant alleles in 26 probands, only one mutant allele in 4 probands, and no mutant alleles in 7 probands. Looking for digenic inheritance, we sequenced the genes encoding the functionally related receptor LRP6, an LRP5 coreceptor FZD4, and an LRP5 ligand, NDP, in the four probands with one mutant allele, and, looking for locus heterogeneity, we sequenced FZD4 and NDP in the seven probands with no mutations, but we found no additional mutations. When we compared clinical features between probands with and without LRP5 mutations, we found no difference in the severity of skeletal disease, prevalence of cognitive impairment, or family history of consanguinity. However, four of the seven probands without detectable mutations had eye pathology that differed from pathology previously described for OPPG. Since many LRP5 mutations are missense changes, to differentiate between a disease-causing mutation and a benign variant, we measured the ability of wild-type and mutant LRP5 to transduce Wnt and Norrin signal ex vivo. Each of the seven OPPG mutations tested, had reduced signal transduction compared with wild-type mutations. These results indicate that early bilateral vitreoretinal eye pathology coupled with skeletal fragility is a strong predictor of LRP5 mutation and that mutations in LRP5 cause OPPG by impairing Wnt and Norrin signal transduction.
Figures

References
Web Resources
-
- Warman lab at Case Western Reserve University, http://genetics.case.edu/warmanlab/ (for the Department of Genetics Web site)
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for OPPG) - PubMed
References
-
- Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F (2003) High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18:960–974 - PubMed
-
- Beighton P, Winship I, Behari D (1985) The ocular form of osteogenesis imperfecta: a new autosomal recessive syndrome. Clin Genet 28:69–75 - PubMed
-
- Bollerslev J, Wilson SG, Dick IM, Islam FM, Ueland T, Palmer L, Devine A, Prince RL (2005) LRP5 gene polymorphisms predict bone mass and incident fractures in elderly Australian women. Bone 36:599–606 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

