Severely incapacitating mutations in patients with extreme short stature identify RNA-processing endoribonuclease RMRP as an essential cell growth regulator
- PMID: 16252239
- PMCID: PMC1271388
- DOI: 10.1086/497708
Severely incapacitating mutations in patients with extreme short stature identify RNA-processing endoribonuclease RMRP as an essential cell growth regulator
Abstract
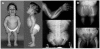
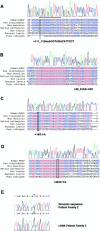
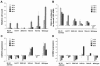
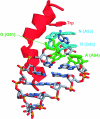
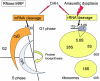
The growth of an individual is deeply influenced by the regulation of cell growth and division, both of which also contribute to a wide variety of pathological conditions, including cancer, diabetes, and inflammation. To identify a major regulator of human growth, we performed positional cloning in an autosomal recessive type of profound short stature, anauxetic dysplasia. Homozygosity mapping led to the identification of novel mutations in the RMRP gene, which was previously known to cause two milder types of short stature with susceptibility to cancer, cartilage hair hypoplasia, and metaphyseal dysplasia without hypotrichosis. We show that different RMRP gene mutations lead to decreased cell growth by impairing ribosomal assembly and by altering cyclin-dependent cell cycle regulation. Clinical heterogeneity is explained by a correlation between the level and type of functional impairment in vitro and the severity of short stature or predisposition to cancer. Whereas the cartilage hair hypoplasia founder mutation affects both pathways intermediately, anauxetic dysplasia mutations do not affect B-cyclin messenger RNA (mRNA) levels but do severely incapacitate ribosomal assembly via defective endonucleolytic cleavage. Anauxetic dysplasia mutations thus lead to poor processing of ribosomal RNA while allowing normal mRNA processing and, therefore, genetically separate the different functions of RNase MRP.
Figures

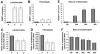

References
Web Resources
-
- ClustalW, http://www.ebi.ac.uk/clustalw/
-
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for RMRP [accession number M29916], human ribosomal DNA complete repeating unit [accession number U13369], CCNB2 [accession number AY864066], and CCNA2 [accession number NM_001237]
-
- GeneSeeker, http://www.cmbi.kun.nl/GeneSeeker/
-
- UCSC Genome Browser, http://genome.ucsc.edu/cgi-bin/hgGateway/
-
- National Center for Biotechnology Information Map Viewer, http://www.ncbi.nlm.nih.gov/mapview/
References
-
- Bonafe L, Schmitt K, Eich G, Giedion A, Superti-Furga A (2002) RMRP gene sequence analysis confirms a cartilage-hair hypoplasia variant with only skeletal manifestations and reveals a high density of single-nucleotide polymorphisms. Clin Genet 61:146–15110.1034/j.1399-0004.2002.610210.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

