Prolonged activation of virus-specific CD8+T cells after acute B19 infection
- PMID: 16253012
- PMCID: PMC1274280
- DOI: 10.1371/journal.pmed.0020343
Prolonged activation of virus-specific CD8+T cells after acute B19 infection
Abstract
Background: Human parvovirus B19 (B19) is a ubiquitous and clinically significant pathogen, causing erythema infectiosum, arthropathy, transient aplastic crisis, and intrauterine fetal death. The phenotype of CD8+ T cells in acute B19 infection has not been studied previously.
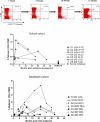
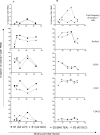
Methods and findings: The number and phenotype of B19-specific CD8+ T cell responses during and after acute adult infection was studied using HLA-peptide multimeric complexes. Surprisingly, these responses increased in magnitude over the first year post-infection despite resolution of clinical symptoms and control of viraemia, with T cell populations specific for individual epitopes comprising up to 4% of CD8+ T cells. B19-specific T cells developed and maintained an activated CD38+ phenotype, with strong expression of perforin and CD57 and downregulation of CD28 and CD27. These cells possessed strong effector function and intact proliferative capacity. Individuals tested many years after infection exhibited lower frequencies of B19-specific cytotoxic T lymphocytes, typically 0.05%-0.5% of CD8+ T cells, which were perforin, CD38, and CCR7 low.
Conclusion: This is the first example to our knowledge of an "acute" human viral infection inducing a persistent activated CD8+ T cell response. The likely explanation--analogous to that for cytomegalovirus infection--is that this persistent response is due to low-level antigen exposure. CD8+ T cells may contribute to the long-term control of this significant pathogen and should be considered during vaccine development.
Conflict of interest statement
Figures



References
-
- van Elsacker-Niele AM, Kroes AC. Human parvovirus B19: Relevance in internal medicine. Neth J Med. 1999;54:221–230. - PubMed
-
- Lundqvist A, Tolfvenstam T, Bostic J, Soderlund M, Broliden K. Clinical and laboratory findings in immunocompetent patients with persistent parvovirus B19 DNA in bone marrow. Scand J Infect Dis. 1999;31:11–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

