Alternative splicing and nonsense-mediated mRNA decay regulate mammalian ribosomal gene expression
- PMID: 16254077
- PMCID: PMC1270949
- DOI: 10.1093/nar/gki905
Alternative splicing and nonsense-mediated mRNA decay regulate mammalian ribosomal gene expression
Abstract
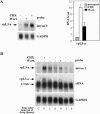
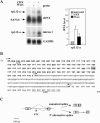
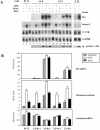
Messenger RNAs containing premature stop codons are generally targeted for degradation through nonsense-mediated mRNA decay (NMD). This mechanism degrades aberrant transcripts derived from mutant genes containing nonsense or frameshift mutations. Wild-type genes also give rise to transcripts targeted by NMD. For example, some wild-type genes give rise to alternatively spliced transcripts that are targeted for decay by NMD. In Caenorhabditis elegans, the ribosomal protein (rp) L12 gene generates a nonsense codon-bearing alternatively spliced transcript that is induced in an autoregulatory manner by the rpL12 protein. By pharmacologically blocking the NMD pathway, we identified alternatively spliced mRNA transcripts derived from the human rpL3 and rpL12 genes that are natural targets of NMD. The deduced protein sequence of these alternatively spliced transcripts suggests that they are unlikely to encode functional ribosomal proteins. Overexpression of rpL3 increased the level of the alternatively spliced rpL3 mRNA and decreased the normally expressed rpL3. This indicates that rpL3 regulates its own production by a negative feedback loop and suggests the possibility that NMD participates in this regulatory loop by degrading the non-functional alternatively spliced transcript.
Figures



References
-
- Wagner E., Lykke-Andersen J. mRNA surveillance: the perfect persist. J. Cell Sci. 2002;115:3033–3038. - PubMed
-
- Culbertson M.R., Leeds P.F. Looking at mRNA decay pathways through the window of molecular evolution. Curr. Opin. Genet. Dev. 2003;13:207–214. - PubMed
-
- Gonzalez C.I., Wang W., Peltz S.W. Nonsense-mediated mRNA decay in Saccharomyces cerevisiae: a quality control mechanism that degrades transcripts harboring premature termination codons. Cold Spring Harb. Symp. Quant. Biol. 2001;66:321–328. - PubMed
-
- Ruiz-Echevarria M.J., Czaplinski K., Peltz S.W. Making sense of nonsense in yeast. Trends Biochem. Sci. 1996;21:433–438. - PubMed
-
- Maquat L.E. Nonsense-mediated RNA decay in mammalian cells: A splicing dependent means to down-regulate the levels of mRNAs that prematurely terminate translation. In: Sonenberg N., Hershey J.W.B., Mathews M.B., editors. Translation Control of Gene Expression. NY: Cold Spring Harbor; 2000. pp. 849–868.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

