Activation of the extracellular signal regulated kinase (ERK) pathway in human melanoma
- PMID: 16254105
- PMCID: PMC1770768
- DOI: 10.1136/jcp.2005.025957
Activation of the extracellular signal regulated kinase (ERK) pathway in human melanoma
Abstract
Background: Several studies suggest that melanoma may be resistant to treatment because of resistance to apoptosis and that this may be the result of activation of the extracellular signal regulated kinase (ERK1/2) pathway.
Aims: To test this hypothesis by examining the expression of ERK1/2 and its activated form in histological sections of melanoma and its relation to known prognostic features of the disease.
Materials/methods: Immunohistochemistry with antibodies to ERK1/2 and phosphorylated ERK (p-ERK) was performed on formalin fixed sections from 42 primary melanomas, 38 metastases, and 20 naevi. Fourteen of the primary melanomas were in the radial and 28 in the vertical growth phase.
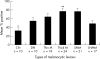
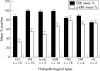
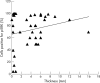
Results: ERK1/2 was widely expressed (100%) in all the (pigmented) lesions studied. p-ERK1/2 expression was much lower in compound (32.4%) and dysplastic (54.5%) naevi than in primary melanoma (nodular 78.8%, superficial spreading 67%) and subcutaneous metastases (76.3%). p-ERK expression was much lower in lymph node metastases (48.5%), suggesting that the microenvironment may influence the activation of ERK. There was a (non-significant) trend for p-ERK expression to be higher in thick (>1.0 mm) versus thin (< or =1.0 mm) melanoma (p = 0.23). There was a trend for overall survival to be related to p-ERK expression in patients with melanoma over 1 mm in thickness.
Conclusions: Expression of activated ERK1/2 in melanocytic lesions appears to be related to malignant potential so that activation of ERK1/2 may be important in melanoma progression. These results provide important histological support for the proposal that inhibition of this signalling pathway may be useful in treatment of melanoma.
Figures


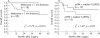
Similar articles
-
Activation of c-jun N-terminal kinase is associated with cell proliferation and shorter relapse-free period in superficial spreading malignant melanoma.Mod Pathol. 2006 Nov;19(11):1446-55. doi: 10.1038/modpathol.3800662. Epub 2006 Sep 1. Mod Pathol. 2006. PMID: 16951673
-
Inducible nitric oxide synthase expression in benign and malignant cutaneous melanocytic lesions.J Pathol. 2001 Jun;194(2):194-200. doi: 10.1002/1096-9896(200106)194:2<194::AID-PATH851>3.0.CO;2-S. J Pathol. 2001. PMID: 11400148
-
Expression of activated extracellular signal-regulated kinases 1/2 in malignant melanomas: relationship with clinical outcome.Clin Cancer Res. 2003 Nov 1;9(14):5325-31. Clin Cancer Res. 2003. PMID: 14614017
-
ERK1/2 is highly phosphorylated in melanoma metastases and protects melanoma cells from cisplatin-mediated apoptosis.J Invest Dermatol. 2007 Sep;127(9):2207-15. doi: 10.1038/sj.jid.5700870. Epub 2007 May 17. J Invest Dermatol. 2007. PMID: 17508026
-
The fatty acid binding protein 7 (FABP7) is involved in proliferation and invasion of melanoma cells.BMC Cancer. 2008 Sep 30;8:276. doi: 10.1186/1471-2407-8-276. BMC Cancer. 2008. PMID: 18826602 Free PMC article.
Cited by
-
Specifically targeting ERK1 or ERK2 kills melanoma cells.J Transl Med. 2012 Jan 25;10:15. doi: 10.1186/1479-5876-10-15. J Transl Med. 2012. PMID: 22277029 Free PMC article.
-
CD24 induces the invasion of cholangiocarcinoma cells by upregulating CXCR4 and increasing the phosphorylation of ERK1/2.Oncol Lett. 2013 Nov;6(5):1439-1446. doi: 10.3892/ol.2013.1587. Epub 2013 Sep 17. Oncol Lett. 2013. PMID: 24179538 Free PMC article.
-
Etlingera elatior Extract promotes cell death in B16 melanoma cells via down-regulation of ERK and Akt signaling pathways.BMC Complement Altern Med. 2017 Aug 22;17(1):415. doi: 10.1186/s12906-017-1921-y. BMC Complement Altern Med. 2017. PMID: 28830513 Free PMC article.
-
Melanocytic nevi and melanoma: unraveling a complex relationship.Oncogene. 2017 Oct 19;36(42):5771-5792. doi: 10.1038/onc.2017.189. Epub 2017 Jun 12. Oncogene. 2017. PMID: 28604751 Free PMC article. Review.
-
Matched three-dimensional organoids and two-dimensional cell lines of melanoma brain metastases mirror response to targeted molecular therapy.Sci Rep. 2024 Oct 22;14(1):24843. doi: 10.1038/s41598-024-76583-8. Sci Rep. 2024. PMID: 39438602 Free PMC article.
References
-
- Armstrong B. Epidemiology of cutaneous melanoma and current trends. London: Martin Dunitz, 2004.
-
- Coates M, Tracey E. Cancer in New South Wales: incidence and mortality 1997. N S W Public Health Bull 2001;12:40–2. - PubMed
-
- Azzola MF, Shaw HM, Thompson JF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer 2003;97:1488–98. - PubMed
-
- Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001;19:3622–34. - PubMed
-
- McKinnon JG, Yu XQ, McCarthy WH, et al. Prognosis for patients with thin cutaneous melanoma: long-term survival data from New South Wales Central Cancer Registry and the Sydney Melanoma Unit. Cancer 2003;98:1223–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
