NS1 protein secretion during the acute phase of West Nile virus infection
- PMID: 16254328
- PMCID: PMC1280181
- DOI: 10.1128/JVI.79.22.13924-13933.2005
NS1 protein secretion during the acute phase of West Nile virus infection
Abstract
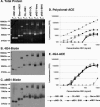
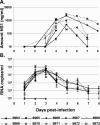
The West Nile virus (WNV) nonstructural protein NS1 is a protein of unknown function that is found within, associated with, and secreted from infected cells. We systematically investigated the kinetics of NS1 secretion in vitro and in vivo to determine the potential use of this protein as a diagnostic marker and to analyze NS1 secretion in relation to the infection cycle. A sensitive antigen capture enzyme-linked immunosorbent assay (ELISA) for detection of WNV NS1 (polyclonal-ACE) was developed, as well as a capture ELISA for the specific detection of NS1 multimers (4G4-ACE). The 4G4-ACE detected native NS1 antigens at high sensitivity, whereas the polyclonal-ACE had a higher specificity for recombinant forms of the protein. Applying these assays we found that only a small fraction of intracellular NS1 is secreted and that secretion of NS1 in tissue culture is delayed compared to the release of virus particles. In experimentally infected hamsters, NS1 was detected in the serum between days 3 and 8 postinfection, peaking on day 5, the day prior to the onset of clinical disease; immunoglobulin M (IgM) antibodies were detected at low levels on day 5 postinfection. Although real-time PCR gave the earliest indication of infection (day 1), the diagnostic performance of the 4G4-ACE was comparable to that of real-time PCR during the time period when NS1 was secreted. Moreover, the 4G4-ACE was found to be superior in performance to both the IgM and plaque assays during this time period, suggesting that NS1 is a viable early diagnostic marker of WNV infection.
Figures
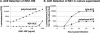
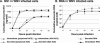


Similar articles
-
Development of a double antibody sandwich ELISA for West Nile virus detection using monoclonal antibodies against non-structural protein 1.PLoS One. 2014 Oct 10;9(10):e108623. doi: 10.1371/journal.pone.0108623. eCollection 2014. PLoS One. 2014. PMID: 25303282 Free PMC article.
-
Remodeling of the Actin Network Associated with the Non-Structural Protein 1 (NS1) of West Nile Virus and Formation of NS1-Containing Tunneling Nanotubes.Viruses. 2019 Sep 27;11(10):901. doi: 10.3390/v11100901. Viruses. 2019. PMID: 31569658 Free PMC article.
-
Replication-Defective West Nile Virus with NS1 Deletion as a New Vaccine Platform for Flavivirus.J Virol. 2019 Aug 13;93(17):e00720-19. doi: 10.1128/JVI.00720-19. Print 2019 Sep 1. J Virol. 2019. PMID: 31189715 Free PMC article.
-
Defining the levels of secreted non-structural protein NS1 after West Nile virus infection in cell culture and mice.J Med Virol. 2008 Mar;80(3):547-56. doi: 10.1002/jmv.21091. J Med Virol. 2008. PMID: 18205232 Free PMC article.
-
Establishment of an Algorithm Using prM/E- and NS1-Specific IgM Antibody-Capture Enzyme-Linked Immunosorbent Assays in Diagnosis of Japanese Encephalitis Virus and West Nile Virus Infections in Humans.J Clin Microbiol. 2016 Feb;54(2):412-22. doi: 10.1128/JCM.02469-15. Epub 2015 Dec 9. J Clin Microbiol. 2016. PMID: 26659204 Free PMC article.
Cited by
-
Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes.Nature. 2017 May 25;545(7655):482-486. doi: 10.1038/nature22365. Epub 2017 May 17. Nature. 2017. PMID: 28514450 Free PMC article.
-
Approaches for the development of rapid serological assays for surveillance and diagnosis of infections caused by zoonotic flaviviruses of the Japanese encephalitis virus serocomplex.J Biomed Biotechnol. 2012;2012:379738. doi: 10.1155/2012/379738. Epub 2012 Apr 18. J Biomed Biotechnol. 2012. PMID: 22570528 Free PMC article. Review.
-
The ORF7b protein of severe acute respiratory syndrome coronavirus (SARS-CoV) is expressed in virus-infected cells and incorporated into SARS-CoV particles.J Virol. 2007 Jan;81(2):718-31. doi: 10.1128/JVI.01691-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079322 Free PMC article.
-
Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion.J Virol. 2006 Oct;80(19):9349-60. doi: 10.1128/JVI.01122-06. J Virol. 2006. PMID: 16973541 Free PMC article. Review. No abstract available.
-
Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism.Cell Rep. 2019 Feb 5;26(6):1598-1613.e8. doi: 10.1016/j.celrep.2019.01.036. Cell Rep. 2019. PMID: 30726741 Free PMC article.
References
-
- Alcon, S., A. Talarmin, M. Debruyne, A. Falconar, V. Deubel, and M. Flamand. 2002. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376-381. - PMC - PubMed
-
- Armitage, P., and G. Berry. 1994. Statistical methods in medical research, 3rd ed. Blackwell Scientific Publications, Boston, Mass.
-
- Beaty, B., C. H. Calisher, and R. E. Shope. 1989. Arboviruses, p. 797-855. In N. J. Schmidt and R. W. Emmons (ed.), Diagnostic procedures for viral, rickettsial and chlamydial infections, 6th ed. American Public Health Association, Washington, D.C.
-
- Blitvich, B. J., J. S. Mackenzie, R. J. Coelen, M. J. Howard, and R. A. Hall. 1995. A novel complex formed between the flavivirus E and NS1 proteins: analysis of its structure and function. Arch. Virol. 140:145-156. - PubMed
-
- Blitvich, B. J., D. Scanlon, B. J. Shiell, J. S. Mackenzie, and R. A. Hall. 1999. Identification and analysis of truncated and elongated species of the flavivirus NS1 protein. Virus Res. 60:67-79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

