Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model
- PMID: 16254335
- PMCID: PMC1280209
- DOI: 10.1128/JVI.79.22.13993-14003.2005
Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model
Abstract
Most Epstein-Barr virus (EBV)-positive tumor cells contain one of the latent forms of viral infection. The role of lytic viral gene expression in EBV-associated malignancies is unknown. Here we show that EBV mutants that cannot undergo lytic viral replication are defective in promoting EBV-mediated lymphoproliferative disease (LPD). Early-passage lymphoblastoid cell lines (LCLs) derived from EBV mutants with a deletion of either viral immediate-early gene grew similarly to wild-type (WT) virus LCLs in vitro but were deficient in producing LPD when inoculated into SCID mice. Restoration of lytic EBV gene expression enhanced growth in SCID mice. Acyclovir, which prevents lytic viral replication but not expression of early lytic viral genes, did not inhibit the growth of WT LCLs in SCID mice. Early-passage LCLs derived from the lytic-defective viruses had substantially decreased expression of the cytokine interleukin-6 (IL-6), and restoration of lytic gene expression reversed this defect. Expression of cellular IL-10 and viral IL-10 was also diminished in lytic-defective LCLs. These results suggest that lytic EBV gene expression contributes to EBV-associated lymphoproliferative disease, potentially through induction of paracrine B-cell growth factors.
Figures
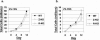





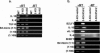

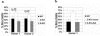
References
-
- Baiocchi, R. A., M. E. Ross, J. C. Tan, C. C. Chou, L. Sullivan, S. Haldar, M. Monne, M. V. Seiden, S. K. Narula, J. Sklar, et al. 1995. Lymphomagenesis in the SCID-hu mouse involves abundant production of human interleukin-10. Blood 85:1063-1074. - PubMed
-
- Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, and E. A. Mesri. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. - PubMed
-
- Beatty, P. R., S. M. Krams, and O. M. Martinez. 1997. Involvement of IL-10 in the autonomous growth of EBV-transformed B cell lines. J. Immunol. 158:4045-4051. - PubMed
-
- Cordano, P., A. Lake, L. Shield, G. M. Taylor, F. E. Alexander, P. R. Taylor, J. White, and R. F. Jarrett. 2005. Effect of IL-6 promoter polymorphism on incidence and outcome in Hodgkin's lymphoma. Br. J. Haematol. 128: 493-495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

