Phosphorylation of the herpes simplex virus tegument protein VP22 has no effect on incorporation of VP22 into the virus but is involved in optimal expression and virion packaging of ICP0
- PMID: 16254340
- PMCID: PMC1280190
- DOI: 10.1128/JVI.79.22.14057-14068.2005
Phosphorylation of the herpes simplex virus tegument protein VP22 has no effect on incorporation of VP22 into the virus but is involved in optimal expression and virion packaging of ICP0
Abstract
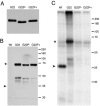
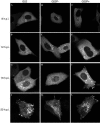
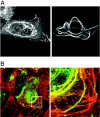
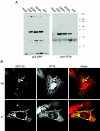
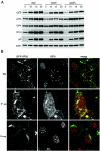
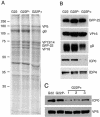
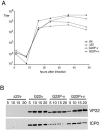
Herpes simplex virus VP22 is a major tegument protein of unknown function. Very recently, we reported that the predominant effect of deleting the VP22 gene was on the expression, localization, and virion incorporation of ICP0. In addition, the Delta22 virus replicated poorly in epithelial MDBK cells. We have also previously shown that VP22 interacts with the tegument protein VP16 and the cellular microtubule network. While the majority of VP22 in infected cells is highly phosphorylated, the nonphosphorylated form of VP22 is the predominant species in the virion, suggesting a differential requirement for phosphorylation through virus replication. Hence, to study the significance of VP22 phosphorylation, we have now constructed two recombinant viruses expressing green fluorescent protein-VP22 (G22) in which the previously identified serine phosphorylation sites have been mutated either to alanine to abolish the phosphorylation status of VP22 (G22P-) or to glutamic acid to mimic permanent phosphorylation (G22P+). Localization studies indicated that the G22P- protein associated tightly with microtubules in some infected cells, suggesting that VP22 phosphorylation may control its interaction with the microtubule network. By contrast, VP22 phosphorylation had no effect on its ability to interact with VP16 and, importantly, had no effect on the relative packaging of VP22. Intriguingly, virion packaging of ICP0 was reduced in the G22P+ virus while ICP0 expression was reduced in the G22P- virus, suggesting that these two ICP0 defects, previously observed in the Delta22 virus, were attributable to different forms of VP22. Furthermore, the Delta22 virus replication defect in MDBK cells correlated with the expression of constitutively charged VP22 in the G22P+ virus. Taken together, these results suggest an important role for VP22 phosphorylation in its relationship with ICP0.
Figures
Similar articles
-
Deletion of the herpes simplex virus VP22-encoding gene (UL49) alters the expression, localization, and virion incorporation of ICP0.J Virol. 2005 Aug;79(15):9735-45. doi: 10.1128/JVI.79.15.9735-9745.2005. J Virol. 2005. PMID: 16014935 Free PMC article.
-
A network of protein interactions around the herpes simplex virus tegument protein VP22.J Virol. 2012 Dec;86(23):12971-82. doi: 10.1128/JVI.01913-12. Epub 2012 Sep 19. J Virol. 2012. PMID: 22993164 Free PMC article.
-
Functional analysis of transcriptional regulation of herpes simplex virus type 1 tegument protein VP22.Sci China C Life Sci. 2008 Nov;51(11):966-72. doi: 10.1007/s11427-008-0127-4. Epub 2008 Nov 7. Sci China C Life Sci. 2008. PMID: 18989638
-
Association of the herpes simplex virus major tegument structural protein VP22 with chromatin.Biochim Biophys Acta. 2010 Mar-Apr;1799(3-4):200-6. doi: 10.1016/j.bbagrm.2009.08.002. Epub 2009 Aug 12. Biochim Biophys Acta. 2010. PMID: 19682615 Review.
-
Alphaherpesvirus Major Tegument Protein VP22: Its Precise Function in the Viral Life Cycle.Front Microbiol. 2020 Aug 7;11:1908. doi: 10.3389/fmicb.2020.01908. eCollection 2020. Front Microbiol. 2020. PMID: 32849477 Free PMC article. Review.
Cited by
-
Regulation and function of phosphorylation on VP8, the major tegument protein of bovine herpesvirus 1.J Virol. 2015 Apr;89(8):4598-611. doi: 10.1128/JVI.03180-14. Epub 2015 Feb 11. J Virol. 2015. PMID: 25673708 Free PMC article.
-
The varicella-zoster virus (VZV) ORF9 protein interacts with the IE62 major VZV transactivator.J Virol. 2007 Jan;81(2):761-74. doi: 10.1128/JVI.01274-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079304 Free PMC article.
-
Post-translational modifications as a key mechanism for herpes simplex virus type I evasion of host innate immunity.Front Microbiol. 2025 Feb 11;16:1543676. doi: 10.3389/fmicb.2025.1543676. eCollection 2025. Front Microbiol. 2025. PMID: 40008039 Free PMC article. Review.
-
Virion incorporation of the herpes simplex virus type 1 tegument protein VP22 occurs via glycoprotein E-specific recruitment to the late secretory pathway.J Virol. 2009 May;83(10):5204-18. doi: 10.1128/JVI.00069-09. Epub 2009 Mar 11. J Virol. 2009. PMID: 19279114 Free PMC article.
-
VP22 core domain from Herpes simplex virus 1 reveals a surprising structural conservation in both the Alpha- and Gammaherpesvirinae subfamilies.J Gen Virol. 2015 Jun;96(Pt 6):1436-1445. doi: 10.1099/vir.0.000078. Epub 2015 Feb 24. J Gen Virol. 2015. PMID: 26068188 Free PMC article.
References
-
- Banham, A. H., and G. L. Smith. 1992. Vaccinia virus gene B1R encodes a 34-kDa serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology 191:803-812. - PubMed
-
- Cambiazo, V., E. Logarinho, H. Pottstock, and C. E. Sunkel. 2000. Microtubule binding of the drosophila DMAP-85 protein is regulated by phosphorylation in vitro. FEBS Lett. 483:37-42. - PubMed
-
- Chi, J. H., C. A. Harley, A. Mukhopadhyay, and D. W. Wilson. 2005. The cytoplasmic tail of herpes simplex virus envelope glycoprotein D binds to the tegument protein VP22 and to capsids. J. Gen. Virol. 86:253-261. - PubMed
-
- Coulter, L. J., H. W. Moss, J. Lang, and D. J. McGeoch. 1993. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J. Gen. Virol. 74:387-395. - PubMed
-
- Cunningham, C., A. J. Davison, A. Dolan, M. C. Frame, D. J. McGeoch, D. M. Meredith, H. W. Moss, and A. C. Orr. 1992. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J. Gen. Virol. 73:303-311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

