Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal Ganglia at the single-cell level
- PMID: 16254342
- PMCID: PMC1280223
- DOI: 10.1128/JVI.79.22.14079-14087.2005
Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal Ganglia at the single-cell level
Abstract
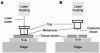
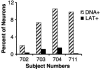
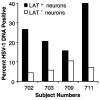
There remains uncertainty and some controversy about the percentages and types of cells in human sensory nerve ganglia that harbor latent herpes simplex virus 1 (HSV-1) and varicella-zoster virus (VZV) DNA. We developed and validated laser-capture microdissection and real-time PCR (LCM/PCR) assays for the presence and copy numbers of HSV-1 gG and VZV gene 62 sequences in single cells recovered from sections of human trigeminal ganglia (TG) obtained at autopsy. Among 970 individual sensory neurons from five subjects, 2.0 to 10.5% were positive for HSV-1 DNA, with a median of 11.3 copies/positive cell, compared with 0.2 to 1.5% of neurons found to be positive by in situ hybridization (ISH) for HSV-1 latency-associated transcripts (LAT), the classical surrogate marker for HSV latency. This indicates a more pervasive latent HSV-1 infection of human TG neurons than originally thought. Combined ISH/LCM/PCR assays revealed that the majority of the latently infected neurons do not accumulate LAT to detectable levels. We detected VZV DNA in 1.0 to 6.9% of individual neurons from 10 subjects. Of the total 1,722 neurons tested, 4.1% were VZV DNA positive, with a median of 6.9 viral genomes/positive cell. After removal by LCM of all visible neurons on a slide, all surrounding nonneuronal cells were harvested and assayed: 21 copies of HSV-1 DNA were detected in approximately 5,200 nonneuronal cells, while nine VZV genomes were detected in approximately 14,200 nonneuronal cells. These data indicate that both HSV-1 and VZV DNAs persist in human TG primarily, if not exclusively, in a moderate percentage of neuronal cells.
Figures

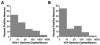

Similar articles
-
Detection of varicella-zoster virus DNA in 414 human trigeminal ganglia from cadavers by the polymerase chain reaction: a comparison of the detection rate of varicella-zoster virus and herpes simplex virus type 1.J Med Virol. 2010 Feb;82(2):345-9. doi: 10.1002/jmv.21687. J Med Virol. 2010. PMID: 20029810
-
Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR.J Virol. 2000 Dec;74(24):11464-71. doi: 10.1128/jvi.74.24.11464-11471.2000. J Virol. 2000. PMID: 11090142 Free PMC article.
-
Viable herpes simplex virus type 1 and varicella-zoster virus in the trigeminal ganglia of human cadavers.J Med Virol. 2013 May;85(5):833-8. doi: 10.1002/jmv.23527. Epub 2013 Feb 27. J Med Virol. 2013. PMID: 23447061
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
-
Use of laser capture microdissection together with in situ hybridization and real-time PCR to study distribution of latent herpes simplex virus genomes in mouse trigeminal ganglia.Methods Mol Biol. 2005;293:285-93. doi: 10.1385/1-59259-853-6:285. Methods Mol Biol. 2005. PMID: 16028427 Review.
Cited by
-
Rapid localized spread and immunologic containment define Herpes simplex virus-2 reactivation in the human genital tract.Elife. 2013 Apr 16;2:e00288. doi: 10.7554/eLife.00288. Elife. 2013. PMID: 23606943 Free PMC article.
-
In vivo disruption of latent HSV by designer endonuclease therapy.JCI Insight. 2016 Sep 8;1(14):e88468. doi: 10.1172/jci.insight.88468. JCI Insight. 2016. PMID: 27642635 Free PMC article.
-
Varicella-zoster virus-specific enzyme-linked immunospot assay responses and zoster-associated pain in herpes zoster subjects.Clin Vaccine Immunol. 2012 Sep;19(9):1411-5. doi: 10.1128/CVI.00095-12. Epub 2012 Jul 11. Clin Vaccine Immunol. 2012. PMID: 22787198 Free PMC article.
-
A deadly dance: the choreography of host-pathogen interactions, as revealed by single-cell technologies.Nat Commun. 2018 Nov 6;9(1):4638. doi: 10.1038/s41467-018-06214-0. Nat Commun. 2018. PMID: 30401874 Free PMC article. Review.
-
Expression of herpes simplex virus 1-encoded microRNAs in human trigeminal ganglia and their relation to local T-cell infiltrates.J Virol. 2011 Oct;85(19):9680-5. doi: 10.1128/JVI.00874-11. Epub 2011 Jul 27. J Virol. 2011. PMID: 21795359 Free PMC article.
References
-
- Burke, R. L., K. Hartog, K. D. Croen, and J. M. Ostrove. 1991. Detection and characterization of latent HSV RNA by in situ and Northern blot hybridization in guinea pigs. Virology 181:793-797. - PubMed
-
- Cai, G. Y., L. I. Pizer, and M. J. Levin. 2002. Fractionation of neurons and satellite cells from human sensory ganglia in order to study herpesvirus latency. J. Virol. Methods 104:21-32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

