Epstein-Barr virus mRNA export factor EB2 is essential for intranuclear capsid assembly and production of gp350
- PMID: 16254345
- PMCID: PMC1280191
- DOI: 10.1128/JVI.79.22.14102-14111.2005
Epstein-Barr virus mRNA export factor EB2 is essential for intranuclear capsid assembly and production of gp350
Abstract
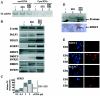
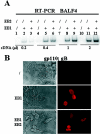
Most human herpesviruses, including Epstein-Barr virus (EBV), express a protein which functions primarily as an mRNA export factor. Previously, we deleted the gene for the Epstein-Barr virus mRNA export factor EB2 from the EBV genome and then introduced the mutated genome into 293 cells. Using a transcomplementation assay in which ectopic expression of the transcription factor EB1/ZEBRA was sufficient to induce the EBV productive cycle, we showed that Ori-Lyt-dependent replication of the EBV DNA occurs in the absence of EB2, indicating that EB2 is not essential for the expression and export of early mRNAs. However, in the absence of EB2, no infectious viral particles are produced (H. Gruffat, J. Batisse, D. Pich, B. Neuhierl, E. Manet, W. Hammerschmidt, and A. Sergeant, J. Virol. 76:9635-9644, 2002). In this report, we now show that EB2 is essential for the nuclear export of most, but not all, late mRNAs produced from intronless genes that translate into proteins involved in intranuclear capsid assembly and maturation. As a consequence, we show that EB2 is essential for the proper assembly of intranuclear capsids. Interestingly, the late BLLF1 gene contains an intron, and both unspliced and spliced mRNAs must be exported to the cytoplasm to be translated into gp350 and gp220, respectively (M. Hummel, D. A. Thorley-Lawson, and E. Kieff, J. Virol. 49:413-417, 1984). Our results also demonstrate that although BLLF1 spliced mRNAs are exported to the cytoplasm independently of EB2, EB2 is essential for the nuclear export of unspliced BLLF1 mRNA. In the same assay, herpes simplex virus 1 ICP27 completely inhibited the nuclear export of BLLF1 spliced mRNAs whereas unspliced BLLF1 mRNAs were exported, confirming that in a physiological assay, ICP27 inhibits splicing.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

