Characterization of Parvovirus B19 genotype 2 in KU812Ep6 cells
- PMID: 16254355
- PMCID: PMC1280213
- DOI: 10.1128/JVI.79.22.14197-14206.2005
Characterization of Parvovirus B19 genotype 2 in KU812Ep6 cells
Abstract
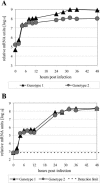
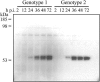
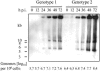
An infectious parvovirus B19 (B19V) genotype 2 variant was identified as a high-titer contaminant in a human plasma donation. Genome analysis revealed a 138-bp insertion within the p6 promoter. The inserted sequence was represented by an additional 30 bp from the end of the inverted terminal repeat adjacent to a 108-bp element found also, in inverted orientation, at the extreme right end of the unique sequence of the genome. However, despite the profound variations in the promoter region, the pattern of gene expression and DNA replication did not differ between genotype 1 and genotype 2 in permissive erythroid KU812Ep6 cells. Capsid proteins of both genotypes differ in their amino acid sequences. However, equivalent kinetics of virus inactivation at 56 degrees C or pH 4 indicated a comparable physicochemical stability of virus capsids. Sera from six individuals infected by B19V genotype 1 were investigated on cross-neutralization of B19V genotype 2 in vitro. Similar neutralization of both B19V genotypes was observed in sera from three individuals, while the sera from three other individuals showed weaker cross-neutralization for genotype 2. In conclusion, the in vitro replication characteristics and physical stability of B19V capsids are very similar between human parvovirus B19 genotypes 1 and 2, and cross-neutralization indicates a close antigenic relation of genotypes 1 and 2.
Figures




Similar articles
-
Molecular characterization of human parvovirus B19 genotypes 2 and 3.Virology. 2009 Nov 25;394(2):276-85. doi: 10.1016/j.virol.2009.08.044. Epub 2009 Sep 15. Virology. 2009. PMID: 19758675 Free PMC article.
-
Existence of various human parvovirus B19 genotypes in Chinese plasma pools: genotype 1, genotype 3, putative intergenotypic recombinant variants and new genotypes.Virol J. 2016 Sep 17;13(1):155. doi: 10.1186/s12985-016-0611-6. Virol J. 2016. PMID: 27639978 Free PMC article.
-
Inactivation and neutralization of parvovirus B19 Genotype 3.Transfusion. 2012 Jul;52(7):1490-7. doi: 10.1111/j.1537-2995.2012.03573.x. Epub 2012 Feb 17. Transfusion. 2012. PMID: 22339291
-
Update of the human parvovirus B19 biology.Transfus Clin Biol. 2016 Feb;23(1):5-12. doi: 10.1016/j.tracli.2015.11.006. Epub 2016 Jan 6. Transfus Clin Biol. 2016. PMID: 26778837 Review.
-
Genotypes of erythrovirus B19, their geographical distribution & circulation in cases with various clinical manifestations.Indian J Med Res. 2018 Mar;147(3):239-247. doi: 10.4103/ijmr.IJMR_1816_16. Indian J Med Res. 2018. PMID: 29923512 Free PMC article. Review.
Cited by
-
Human Parvoviruses.Clin Microbiol Rev. 2017 Jan;30(1):43-113. doi: 10.1128/CMR.00040-16. Clin Microbiol Rev. 2017. PMID: 27806994 Free PMC article. Review.
-
Biological and immunological relations among human parvovirus B19 genotypes 1 to 3.J Virol. 2007 Jul;81(13):6927-35. doi: 10.1128/JVI.02713-06. Epub 2007 Apr 4. J Virol. 2007. PMID: 17409158 Free PMC article.
-
Bioportfolio: lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue.Proc Natl Acad Sci U S A. 2006 May 9;103(19):7450-3. doi: 10.1073/pnas.0602259103. Epub 2006 May 1. Proc Natl Acad Sci U S A. 2006. PMID: 16651522 Free PMC article.
-
Prevalence and Phylogenetic Analysis of Parvovirus (B19V) among Blood Donors with Different Nationalities Residing in Qatar.Viruses. 2021 Mar 24;13(4):540. doi: 10.3390/v13040540. Viruses. 2021. PMID: 33805034 Free PMC article.
-
Genetic variants of human parvovirus B19 in South Africa: cocirculation of three genotypes and identification of a novel subtype of genotype 1.J Clin Microbiol. 2010 Jan;48(1):137-42. doi: 10.1128/JCM.00610-09. Epub 2009 Nov 18. J Clin Microbiol. 2010. PMID: 19923483 Free PMC article.
References
-
- Aberham, C., C. Pendel, P. Gross, G. Zerlauth, and M. Gessner. 2001. A quantitative internally controlled real-time PCR assay for the detection of parvovirus B19 DNA. J. Virol. Methods 92:183-191. - PubMed
-
- Agbandje, M., S. Kajigaya, R. McKenna, N. S. Young, and M. G. Rossmann. 1994. The structure of human parvovirus B19 at 8 A resolution. Virology 203:106-115. - PubMed
-
- Aubin, J. T., C. Defer, M. Vidaud, M. M. Maniez, and B. Flan. 2000. Large-scale screening for human parvovirus B19 DNA by PCR: application to the quality control of plasma for fractionation. Vox Sang. 78:7-12. - PubMed
-
- Azzi, A., M. Morfini, and P. M. Mannucci. 1999. The transfusion-associated transmission of parvovirus B19. Transfus. Med. Rev. 13:194-204. - PubMed
-
- Blümel, J., I. Schmidt, H. Willkommen, and J. Löwer. 2002. Inactivation of parvovirus B19 during pasteurization of human serum albumin. Transfusion 42:1011-1018. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

