Establishment and maintenance of Kaposi's sarcoma-associated herpesvirus latency in B cells
- PMID: 16254372
- PMCID: PMC1280215
- DOI: 10.1128/JVI.79.22.14383-14391.2005
Establishment and maintenance of Kaposi's sarcoma-associated herpesvirus latency in B cells
Abstract
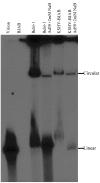
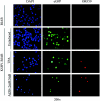
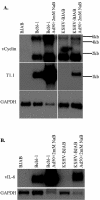
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV) is the infectious cause of Kaposi's sarcoma and is also associated with two B-cell lymphoproliferative diseases, primary effusion lymphoma and the plasmablastic form of multicentric Castleman's disease. KSHV is also found in the B-cell fraction of peripheral blood mononucleocytes of some KS patients. Despite in vivo infection of B cells and the ability of KSHV to infect many cell types in culture, to date B cells in culture have been resistant to KSHV infection. However, as shown here, the lack of infection is not due to the inability of B cells to support latent KSHV infection. When KSHV DNA is introduced into B cells, the virus is maintained as an episome and can establish and maintain latency over the course of months. As in all primary effusion lymphoma cell lines, there is a low level of spontaneous lytic replication in latently infected BJAB cells. Importantly, viral gene expression is similar to that of primary effusion lymphoma cell lines. Furthermore, the virus can be reactivated to higher levels with specific stimuli and transmitted to other cells, indicating that this is a productive infection. Thus B cells in culture are capable of establishing, maintaining, and reactivating from latency. These studies provide a controlled system to analyze how KSHV alters B cells during KSHV latency and reactivation.
Figures



Similar articles
-
Transcriptome analysis of Kaposi's sarcoma-associated herpesvirus during de novo primary infection of human B and endothelial cells.J Virol. 2015 Mar;89(6):3093-111. doi: 10.1128/JVI.02507-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552714 Free PMC article.
-
Efficient infection of a human B cell line with cell-free Kaposi's sarcoma-associated herpesvirus.J Virol. 2014 Feb;88(3):1748-57. doi: 10.1128/JVI.03063-13. Epub 2013 Nov 20. J Virol. 2014. PMID: 24257608 Free PMC article.
-
Generation of high-titre virus stocks using BrK.219, a B-cell line infected stably with recombinant Kaposi's sarcoma-associated herpesvirus.J Virol Methods. 2015 Jun 1;217:79-86. doi: 10.1016/j.jviromet.2015.02.022. Epub 2015 Feb 28. J Virol Methods. 2015. PMID: 25736227
-
KSHV Genome Replication and Maintenance in Latency.Adv Exp Med Biol. 2018;1045:299-320. doi: 10.1007/978-981-10-7230-7_14. Adv Exp Med Biol. 2018. PMID: 29896673 Review.
-
Epigenetic regulation of Kaposi's sarcoma-associated herpesvirus replication.Semin Cancer Biol. 2009 Jun;19(3):153-7. doi: 10.1016/j.semcancer.2009.02.010. Epub 2009 Feb 21. Semin Cancer Biol. 2009. PMID: 19429478 Free PMC article. Review.
Cited by
-
Abortive lytic reactivation of KSHV in CBF1/CSL deficient human B cell lines.PLoS Pathog. 2013;9(5):e1003336. doi: 10.1371/journal.ppat.1003336. Epub 2013 May 16. PLoS Pathog. 2013. PMID: 23696732 Free PMC article.
-
Development of a fluorescence-based assay to screen antiviral drugs against Kaposi's sarcoma associated herpesvirus.Mol Cancer Ther. 2007 Aug;6(8):2360-70. doi: 10.1158/1535-7163.MCT-07-0108. Mol Cancer Ther. 2007. PMID: 17699731 Free PMC article.
-
Activation of Akt through gp130 receptor signaling is required for Kaposi's sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells.J Virol. 2008 Sep;82(17):8771-9. doi: 10.1128/JVI.00766-08. Epub 2008 Jun 25. J Virol. 2008. PMID: 18579585 Free PMC article.
-
BH3 Profiling Reveals Selectivity by Herpesviruses for Specific Bcl-2 Proteins To Mediate Survival of Latently Infected Cells.J Virol. 2015 May;89(10):5739-46. doi: 10.1128/JVI.00236-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740993 Free PMC article.
-
Activation of the B cell antigen receptor triggers reactivation of latent Kaposi's sarcoma-associated herpesvirus in B cells.J Virol. 2013 Jul;87(14):8004-16. doi: 10.1128/JVI.00506-13. Epub 2013 May 15. J Virol. 2013. PMID: 23678173 Free PMC article.
References
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. - PubMed
-
- Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282:245-255. - PubMed
-
- Ambroziak, J. A., D. J. Blackbourn, B. G. Herndier, R. G. Glogau, J. H. Gullett, A. R. McDonald, E. T. Lennette, and J. A. Levy. 1995. Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science 268:582-583. - PubMed
-
- Ansari, M. Q., D. B. Dawson, R. Nador, C. Rutherford, N. R. Schneider, M. J. Latimer, L. Picker, D. M. Knowles, and R. W. McKenna. 1996. Primary body cavity-based AIDS-related lymphomas. Am. J. Clin. Pathol. 105:221-229. - PubMed
-
- Arvanitakis, L., E. A. Mesri, R. G. Nador, J. W. Said, A. S. Asch, D. M. Knowles, and E. Cesarman. 1996. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88:2648-2654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

