Anthrax lethal toxin-mediated killing of human and murine dendritic cells impairs the adaptive immune response
- PMID: 16254597
- PMCID: PMC1266308
- DOI: 10.1371/journal.ppat.0010019
Anthrax lethal toxin-mediated killing of human and murine dendritic cells impairs the adaptive immune response
Abstract
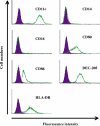
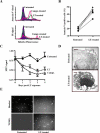
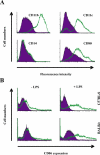
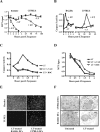
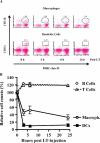
Many pathogens have acquired strategies to combat the immune response. Bacillus anthracis interferes with host defenses by releasing anthrax lethal toxin (LT), which inactivates mitogen-activated protein kinase pathways, rendering dendritic cells (DCs) and T lymphocytes nonresponsive to immune stimulation. However, these cell types are considered resistant to killing by LT. Here we show that LT kills primary human DCs in vitro, and murine DCs in vitro and in vivo. Kinetics of LT-mediated killing of murine DCs, as well as cell death pathways induced, were dependent upon genetic background: LT triggered rapid necrosis in BALB/c-derived DCs, and slow apoptosis in C57BL/6-derived DCs. This is consistent with rapid and slow killing of LT-injected BALB/c and C57BL/6 mice, respectively. We present evidence that anthrax LT impairs adaptive immunity by specifically targeting DCs. This may represent an immune-evasion strategy of the bacterium, and contribute to anthrax disease progression. We also established that genetic background determines whether apoptosis or necrosis is induced by LT. Finally, killing of C57BL/6-derived DCs by LT mirrors that of human DCs, suggesting that C57BL/6 DCs represent a better model system for human anthrax than the prototypical BALB/c macrophages.
Conflict of interest statement
Figures
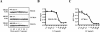
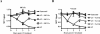
Similar articles
-
Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin.Nature. 2003 Jul 17;424(6946):329-34. doi: 10.1038/nature01794. Nature. 2003. PMID: 12867985
-
Bacillus anthracis lethal toxin attenuates lipoteichoic acid-induced maturation and activation of dendritic cells through a unique mechanism.Mol Immunol. 2009 Oct;46(16):3261-8. doi: 10.1016/j.molimm.2009.08.005. Epub 2009 Aug 31. Mol Immunol. 2009. PMID: 19720398
-
Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis.Cell Cycle. 2007 Mar 15;6(6):758-66. doi: 10.4161/cc.6.6.3991. Epub 2007 Mar 7. Cell Cycle. 2007. PMID: 17374996
-
Immune system paralysis by anthrax lethal toxin: the roles of innate and adaptive immunity.Lancet Infect Dis. 2004 Mar;4(3):166-70. doi: 10.1016/S1473-3099(04)00940-5. Lancet Infect Dis. 2004. PMID: 14998502 Review.
-
Anthrax toxins: A paradigm of bacterial immune suppression.Trends Immunol. 2006 Sep;27(9):434-40. doi: 10.1016/j.it.2006.07.002. Epub 2006 Jul 24. Trends Immunol. 2006. PMID: 16861036 Review.
Cited by
-
Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity.Infect Immun. 2006 Oct;74(10):5840-7. doi: 10.1128/IAI.00712-06. Infect Immun. 2006. PMID: 16988263 Free PMC article.
-
Anthrax toxin uptake by primary immune cells as determined with a lethal factor-beta-lactamase fusion protein.PLoS One. 2009 Nov 23;4(11):e7946. doi: 10.1371/journal.pone.0007946. PLoS One. 2009. PMID: 19956758 Free PMC article.
-
Lethality in a murine model of pulmonary anthrax is reduced by combining nuclear transport modifier with antimicrobial therapy.PLoS One. 2012;7(1):e30527. doi: 10.1371/journal.pone.0030527. Epub 2012 Jan 26. PLoS One. 2012. PMID: 22291977 Free PMC article.
-
Cellular and systemic effects of anthrax lethal toxin and edema toxin.Mol Aspects Med. 2009 Dec;30(6):439-55. doi: 10.1016/j.mam.2009.07.003. Epub 2009 Jul 26. Mol Aspects Med. 2009. PMID: 19638283 Free PMC article. Review.
-
Bacillus anthracis spore movement does not require a carrier cell and is not affected by lethal toxin in human lung models.Microbes Infect. 2016 Oct;18(10):615-626. doi: 10.1016/j.micinf.2016.06.004. Epub 2016 Jun 16. Microbes Infect. 2016. PMID: 27320392 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

