Postnatal phenotype and localization of spinal cord V1 derived interneurons
- PMID: 16255029
- PMCID: PMC2997483
- DOI: 10.1002/cne.20711
Postnatal phenotype and localization of spinal cord V1 derived interneurons
Abstract
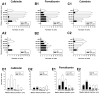
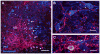
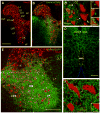
Developmental studies identified four classes (V0, V1, V2, V3) of embryonic interneurons in the ventral spinal cord. Very little is known, however, about their adult phenotypes. Therefore, we characterized the location, neurotransmitter phenotype, calcium-buffering protein expression, and axon distributions of V1-derived neurons in the adult mouse spinal cord. In the mature (P20 and older) spinal cord, most V1-derived neurons are located in lateral LVII and in LIX, few in medial LVII, and none in LVIII. Approximately 40% express calbindin and/or parvalbumin, while few express calretinin. Of seven groups of ventral interneurons identified according to calcium-buffering protein expression, two groups (1 and 4) correspond with V1-derived neurons. Group 1 are Renshaw cells and intensely express calbindin and coexpress parvalbumin and calretinin. They represent 9% of the V1 population. Group 4 express only parvalbumin and represent 27% of V1-derived neurons. V1-derived Group 4 neurons receive contacts from primary sensory afferents and are therefore proprioceptive interneurons. The most ventral neurons in this group receive convergent calbindin-IR Renshaw cell inputs. This subgroup resembles Ia inhibitory interneurons (IaINs) and represents 13% of V1-derived neurons. Adult V1-interneuron axons target LIX and LVII and some enter the deep dorsal horn. V1 axons do not cross the midline. V1-derived axonal varicosities were mostly (>80%) glycinergic and a third were GABAergic. None were glutamatergic or cholinergic. In summary, V1 interneurons develop into ipsilaterally projecting, inhibitory interneurons that include Renshaw cells, Ia inhibitory interneurons, and other unidentified proprioceptive interneurons.
Figures





References
-
- Altman J, Bayer SA. Development of the Human Spinal Cord. Oxford University Press Inc; New York: 2001.
-
- Alvarez FJ, Dewey DE, Harrington DA, Fyffe RE. Cell-type specific organization of glycine receptor clusters in the mammalian spinal cord. J Comp Neurol. 1997;379:150–170. - PubMed
-
- Alvarez FJ, Jonas P, Sapir T, Geiman EJ, Hartley R, Todd AJ, Goulding M. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2003. Postnatal phenotype and localization of spinal cord V1 derived interneurons. Program No. 39.3. Online.
-
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

