Extracellular superoxide dismutase and oxidant damage in osteoarthritis
- PMID: 16255039
- PMCID: PMC2755499
- DOI: 10.1002/art.21387
Extracellular superoxide dismutase and oxidant damage in osteoarthritis
Abstract
Objective: To use human cartilage samples and a mouse model of osteoarthritis (OA) to determine whether extracellular superoxide dismutase (EC-SOD) is a constituent of cartilage and to evaluate whether there is a relationship between EC-SOD deficiency and OA.
Methods: Samples of human cartilage were obtained from femoral heads at the time of joint replacement surgery for OA or femoral neck fracture. Samples of mouse tibial cartilage obtained from STR/ort mice and CBA control mice were compared at 5, 15, and 35 weeks of age. EC-SOD was measured by enzyme-linked immunosorbent assay, Western blotting, and immunohistochemistry techniques. Real-time quantitative reverse transcription-polymerase chain reaction was used to measure messenger RNA for EC-SOD and for endothelial cell, neuronal, and inducible nitric oxide synthases. Nitrotyrosine formation was assayed by Western blotting in mouse cartilage and by fluorescence immunohistochemistry in human cartilage.
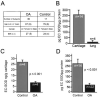
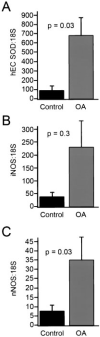
Results: Human articular cartilage contained large amounts of EC-SOD (mean +/- SEM 18.8 +/- 3.8 ng/gm wet weight of cartilage). Cartilage from patients with OA had an approximately 4-fold lower level of EC-SOD compared with cartilage from patients with hip fracture. Young STR/ort mice had decreased levels of EC-SOD in tibial cartilage before histologic evidence of disease occurred, as well as significantly more nitrotyrosine formation at all ages studied.
Conclusion: EC-SOD, the major scavenger of reactive oxygen species in extracellular spaces, is decreased in humans with OA and in an animal model of OA. Our findings suggest that inadequate control of reactive oxygen species plays a role in the pathophysiology of OA.
Figures



Similar articles
-
Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury.Osteoarthritis Cartilage. 2008 Apr;16(4):515-21. doi: 10.1016/j.joca.2007.09.001. Epub 2008 Jan 18. Osteoarthritis Cartilage. 2008. PMID: 18203633
-
Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease.Ann Rheum Dis. 2010 Aug;69(8):1502-10. doi: 10.1136/ard.2009.119966. Epub 2010 May 28. Ann Rheum Dis. 2010. PMID: 20511611 Free PMC article.
-
Chondrocyte death during murine osteoarthritis.Osteoarthritis Cartilage. 2004 Feb;12(2):131-41. doi: 10.1016/j.joca.2003.10.006. Osteoarthritis Cartilage. 2004. PMID: 14723872
-
Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins.Arterioscler Thromb Vasc Biol. 1998 Feb;18(2):157-67. doi: 10.1161/01.atv.18.2.157. Arterioscler Thromb Vasc Biol. 1998. PMID: 9484979 Review.
-
Reactive oxygen species, aging and articular cartilage homeostasis.Free Radic Biol Med. 2019 Feb 20;132:73-82. doi: 10.1016/j.freeradbiomed.2018.08.038. Epub 2018 Aug 31. Free Radic Biol Med. 2019. PMID: 30176344 Free PMC article. Review.
Cited by
-
Aging and osteoarthritis: an inevitable encounter?J Aging Res. 2012;2012:950192. doi: 10.1155/2012/950192. Epub 2012 Jun 7. J Aging Res. 2012. PMID: 22720159 Free PMC article.
-
(Chemical) Roles of HOCl in Rheumatic Diseases.Antioxidants (Basel). 2024 Jul 29;13(8):921. doi: 10.3390/antiox13080921. Antioxidants (Basel). 2024. PMID: 39199167 Free PMC article. Review.
-
Injurious Loading of Articular Cartilage Compromises Chondrocyte Respiratory Function.Arthritis Rheumatol. 2016 Mar;68(3):662-71. doi: 10.1002/art.39460. Arthritis Rheumatol. 2016. PMID: 26473613 Free PMC article.
-
Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix.Osteoarthritis Cartilage. 2009 Aug;17(8):971-9. doi: 10.1016/j.joca.2009.03.002. Epub 2009 Mar 12. Osteoarthritis Cartilage. 2009. PMID: 19303469 Free PMC article. Review.
-
Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration.Sci Rep. 2015 Jun 25;5:11722. doi: 10.1038/srep11722. Sci Rep. 2015. PMID: 26108578 Free PMC article.
References
-
- Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–99. - PubMed
-
- Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I): evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128:179–89. - PubMed
-
- Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets [review] Arthritis Rheum. 2001;44:1237–47. - PubMed
-
- Monboisse JC, Borel JP. Oxidative damage to collagen. EXS. 1992;62:323–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

