Reconstitution and properties of the recombinant glyceraldehyde-3-phosphate dehydrogenase/CP12/phosphoribulokinase supramolecular complex of Arabidopsis
- PMID: 16258009
- PMCID: PMC1283778
- DOI: 10.1104/pp.105.068445
Reconstitution and properties of the recombinant glyceraldehyde-3-phosphate dehydrogenase/CP12/phosphoribulokinase supramolecular complex of Arabidopsis
Abstract
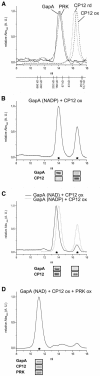
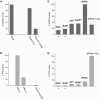
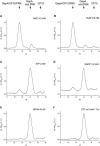
Calvin cycle enzymes glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and phosphoribulokinase (PRK) form together with the regulatory peptide CP12 a supramolecular complex in Arabidopsis (Arabidopsis thaliana) that could be reconstituted in vitro using purified recombinant proteins. Both enzyme activities were strongly influenced by complex formation, providing an effective means for regulation of the Calvin cycle in vivo. PRK and CP12, but not GapA (A(4) isoform of GAPDH), are redox-sensitive proteins. PRK was reversibly inhibited by oxidation. CP12 has no enzymatic activity, but it changed conformation depending on redox conditions. GapA, a bispecific NAD(P)-dependent dehydrogenase, specifically formed a binary complex with oxidized CP12 when bound to NAD. PRK did not interact with either GapA or CP12 singly, but oxidized PRK could form with GapA/CP12 a stable ternary complex of about 640 kD (GapA/CP12/PRK). Exchanging NADP for NAD, reducing CP12, or reducing PRK were all conditions that prevented formation of the complex. Although GapA activity was little affected by CP12 alone, the NADPH-dependent activity of GapA embedded in the GapA/CP12/PRK complex was 80% inhibited in respect to the free enzyme. The NADH activity was unaffected. Upon binding to GapA/CP12, the activity of oxidized PRK dropped from 25% down to 2% the activity of the free reduced enzyme. The supramolecular complex was dissociated by reduced thioredoxins, NADP, 1,3-bisphosphoglycerate (BPGA), or ATP. The activity of GapA was only partially recovered after complex dissociation by thioredoxins, NADP, or ATP, and full GapA activation required BPGA. NADP, ATP, or BPGA partially activated PRK, but full recovery of PRK activity required thioredoxins. The reversible formation of the GapA/CP12/PRK supramolecular complex provides novel possibilities to finely regulate GapA ("non-regulatory" GAPDH isozyme) and PRK (thioredoxin sensitive) in a coordinated manner.
Figures

Similar articles
-
CP12-mediated protection of Calvin-Benson cycle enzymes from oxidative stress.Biochimie. 2014 Feb;97:228-37. doi: 10.1016/j.biochi.2013.10.018. Epub 2013 Nov 5. Biochimie. 2014. PMID: 24211189
-
Co-ordinated gene expression of photosynthetic glyceraldehyde-3-phosphate dehydrogenase, phosphoribulokinase, and CP12 in Arabidopsis thaliana.J Exp Bot. 2005 Jan;56(409):73-80. doi: 10.1093/jxb/eri020. Epub 2004 Nov 8. J Exp Bot. 2005. PMID: 15533878
-
Unravelling the shape and structural assembly of the photosynthetic GAPDH-CP12-PRK complex from Arabidopsis thaliana by small-angle X-ray scattering analysis.Acta Crystallogr D Biol Crystallogr. 2015 Dec 1;71(Pt 12):2372-85. doi: 10.1107/S1399004715018520. Epub 2015 Nov 26. Acta Crystallogr D Biol Crystallogr. 2015. PMID: 26627646
-
Thioredoxin-dependent regulation of photosynthetic glyceraldehyde-3-phosphate dehydrogenase: autonomous vs. CP12-dependent mechanisms.Photosynth Res. 2006 Sep;89(2-3):263-75. doi: 10.1007/s11120-006-9099-z. Epub 2006 Sep 22. Photosynth Res. 2006. PMID: 17031544 Review.
-
Dark complexes of the Calvin-Benson cycle in a physiological perspective.Semin Cell Dev Biol. 2024 Mar 1;155(Pt A):48-58. doi: 10.1016/j.semcdb.2023.03.002. Epub 2023 Mar 6. Semin Cell Dev Biol. 2024. PMID: 36889996 Review.
Cited by
-
The Importance of the C-Terminal Cys Pair of Phosphoribulokinase in Phototrophs in Thioredoxin-Dependent Regulation.Plant Cell Physiol. 2022 Jun 15;63(6):855-868. doi: 10.1093/pcp/pcac050. Plant Cell Physiol. 2022. PMID: 35413120 Free PMC article.
-
Expression analysis of the Arabidopsis CP12 gene family suggests novel roles for these proteins in roots and floral tissues.J Exp Bot. 2008;59(14):3975-85. doi: 10.1093/jxb/ern236. Epub 2008 Oct 28. J Exp Bot. 2008. PMID: 18974062 Free PMC article.
-
Inter-species variation in the oligomeric states of the higher plant Calvin cycle enzymes glyceraldehyde-3-phosphate dehydrogenase and phosphoribulokinase.J Exp Bot. 2011 Jul;62(11):3799-805. doi: 10.1093/jxb/err057. Epub 2011 Apr 15. J Exp Bot. 2011. PMID: 21498632 Free PMC article.
-
Proteome-wide identification of S-sulfenylated cysteines reveals metabolic response to freezing stress after cold acclimation in Brassica napus.Front Plant Sci. 2022 Sep 29;13:1014295. doi: 10.3389/fpls.2022.1014295. eCollection 2022. Front Plant Sci. 2022. PMID: 36275609 Free PMC article.
-
Functional Characterization of Alr0765, A Hypothetical Protein from Anabaena PCC 7120 Involved in Cellular Energy Status Sensing, Iron Acquisition and Abiotic Stress Management in E. coli Using Molecular, Biochemical and Computational Approaches.Curr Genomics. 2020 May;21(4):295-310. doi: 10.2174/1389202921999200424181239. Curr Genomics. 2020. PMID: 33071622 Free PMC article.
References
-
- Baalmann E, Backhausen JE, Rak C, Vetter S, Scheibe R (1995) Reductive modification and non-reductive activation of spinach chloroplast NADP-glyceraldehyde-3-phosphate dehydrogenase. Arch Biochem Biophys 324: 201–208 - PubMed
-
- Baalmann E, Scheibe R, Cerff R, Martin W (1996) Functional studies of chloroplast glyceraldehyde-3-phosphate dehydrogenase subunits A and B expressed in Escherichia coli: formation of highly active A4 and B4 homotetramers and evidence that the aggregation of the B4 complex is mediated by the B-subunit carboxy terminus. Plant Mol Biol 32: 505–513 - PubMed
-
- Brandes HK, Larimer FW, Hartman FC (1996) The molecular pathway for the regulation of phosphoribulokinase by thioredoxin f. J Biol Chem 271: 3333–3335 - PubMed
-
- Brinkmann H, Cerff R, Salomon M, Soll J (1989) Cloning and sequence analysis of cDNAs encoding the cytosolic precursors of subunits GapA and GapB of chloroplast glyceraldehyde-3-phosphate dehydrogenase from pea and spinach. Plant Mol Biol 13: 81–94 - PubMed
-
- Buchanan BB, Balmer Y (2005) Redox regulation: a broadening horizon. Annu Rev Plant Biol 56: 187–220 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

