Utilizing ESEEM spectroscopy to locate the position of specific regions of membrane-active peptides within model membranes
- PMID: 16258052
- PMCID: PMC1367055
- DOI: 10.1529/biophysj.105.062992
Utilizing ESEEM spectroscopy to locate the position of specific regions of membrane-active peptides within model membranes
Abstract
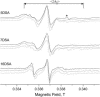
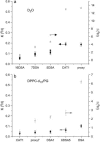
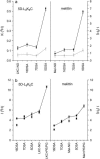
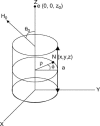
Membrane-active peptides participate in many cellular processes, and therefore knowledge of their mode of interaction with phospholipids is essential for understanding their biological function. Here we present a new methodology based on electron spin-echo envelope modulation to probe, at a relatively high resolution, the location of membrane-bound lytic peptides and to study their effect on the water concentration profile of the membrane. As a first example, we determined the location of the N-terminus of two membrane-active amphipathic peptides, the 26-mer bee venom melittin and a de novo designed 15-mer D,L-amino acid amphipathic peptide (5D-L9K6C), both of which are antimicrobial and bind and act similarly on negatively charged membranes. A nitroxide spin label was introduced to the N-terminus of the peptides and measurements were performed either in H2O solutions with deuterated model membranes or in D2O solutions with nondeuterated model membranes. The lipids used were dipalmitoyl phosphatidylcholine (DPPC) and phosphatidylglycerol (PG), (DPPC/PG (7:3 w/w)), egg phosphatidylcholine (PC) and PG (PC/PG (7:3 w/w)), and phosphatidylethanolamine (PE) and PG (PE/PG, 7:3w/w). The modulation induced by the 2H nuclei was determined and compared with a series of controls that produced a reference "ruler". Actual estimated distances were obtained from a quantitative analysis of the modulation depth based on a simple model of an electron spin situated at a certain distance from the bottom of a layer with homogeneously distributed deuterium nuclei. The N-terminus of both peptides was found to be in the solvent layer in both the DPPC/PG and PC/PG membranes. For PE/PG, a further displacement into the solvent was observed. The addition of the peptides was found to change the water distribution in the membrane, making it "flatter" and increasing the penetration depth into the hydrophobic region.
Figures



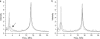

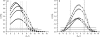


Similar articles
-
Interaction of bee venom melittin with zwitterionic and negatively charged phospholipid bilayers: a spin-label electron spin resonance study.Biophys J. 1997 Feb;72(2 Pt 1):767-78. doi: 10.1016/s0006-3495(97)78711-3. Biophys J. 1997. PMID: 9017202 Free PMC article.
-
Effect of drastic sequence alteration and D-amino acid incorporation on the membrane binding behavior of lytic peptides.Biochemistry. 2004 Jun 1;43(21):6393-403. doi: 10.1021/bi049944h. Biochemistry. 2004. PMID: 15157073
-
Investigation of model membrane disruption mechanism by melittin using pulse electron paramagnetic resonance spectroscopy and cryogenic transmission electron microscopy.J Phys Chem B. 2012 Jan 12;116(1):179-88. doi: 10.1021/jp207159z. Epub 2011 Dec 12. J Phys Chem B. 2012. PMID: 22091896
-
Structure and dynamics of phospholipids in membranes elucidated by combined use of NMR and vibrational spectroscopies.Biochim Biophys Acta Biomembr. 2020 Sep 1;1862(9):183352. doi: 10.1016/j.bbamem.2020.183352. Epub 2020 May 11. Biochim Biophys Acta Biomembr. 2020. PMID: 32407775 Review.
-
Time-resolved electron spin resonance studies of spin-labelled lipids in membranes.Chem Phys Lipids. 2006 Jun;141(1-2):142-57. doi: 10.1016/j.chemphyslip.2006.02.009. Epub 2006 Mar 13. Chem Phys Lipids. 2006. PMID: 16564516 Review.
Cited by
-
Electron Paramagnetic Resonance and Electron Spin Echo Studies of Co2+ Coordination by Nicotinamide Adenine Dinucleotide (NAD+) in Water Solution.Appl Magn Reson. 2013 Jul;44(7):817-826. doi: 10.1007/s00723-013-0444-z. Epub 2013 Feb 24. Appl Magn Reson. 2013. PMID: 23766555 Free PMC article.
-
Solid-state NMR paramagnetic relaxation enhancement immersion depth studies in phospholipid bilayers.J Magn Reson. 2010 Nov;207(1):89-94. doi: 10.1016/j.jmr.2010.08.012. Epub 2010 Aug 24. J Magn Reson. 2010. PMID: 20851650 Free PMC article.
-
Electron Spin Relaxation of Photoexcited Porphyrin in Water-Glycerol Glass.Molecules. 2020 Jun 9;25(11):2677. doi: 10.3390/molecules25112677. Molecules. 2020. PMID: 32527023 Free PMC article.
-
Probing water density and dynamics in the chaperonin GroEL cavity.J Am Chem Soc. 2014 Jul 2;136(26):9396-403. doi: 10.1021/ja503501x. Epub 2014 Jun 20. J Am Chem Soc. 2014. PMID: 24888581 Free PMC article.
-
Application of DNP-enhanced solid-state NMR to studies of amyloid-β peptide interaction with lipid membranes.Chem Phys Lipids. 2021 May;236:105071. doi: 10.1016/j.chemphyslip.2021.105071. Epub 2021 Mar 11. Chem Phys Lipids. 2021. PMID: 33716023 Free PMC article.
References
-
- Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61–92. - PubMed
-
- Nicolas, P., and A. Mor. 1995. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 49:277–304. - PubMed
-
- Hancock, R. E., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402–410. - PubMed
-
- Brotz, H., and H. G. Sahl. 2000. New insights into the mechanism of action of lantibiotics: diverse biological effects by binding to the same molecular target. J. Antimicrob. Chemother. 46:1–6. - PubMed
-
- Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature. 415:389–395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

