Intronic regulation of matrix metalloproteinase-2 revealed by in vivo transcriptional analysis in ischemia
- PMID: 16258061
- PMCID: PMC1283457
- DOI: 10.1073/pnas.0508085102
Intronic regulation of matrix metalloproteinase-2 revealed by in vivo transcriptional analysis in ischemia
Abstract
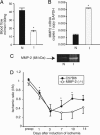
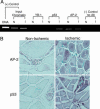
Matrix metalloproteinase-2 (MMP-2) plays an essential role in angiogenesis and arteriogenesis, two processes critical to restoration of tissue perfusion after ischemia. MMP-2 expression is increased in tissue ischemia, but the responsible mechanisms remain unknown. We studied the transcriptional activation of the MMP-2 gene in a model of hindlimb ischemia by using various MMP-2-lacZ reporter mice and chromatin immunoprecipitation. MMP-2 activity and mRNA were increased after hindlimb ischemia. Mice with targeted deletion of MMP-2 had impaired restoration of perfusion and a high incidence of limb gangrene, indicating that MMP-2 plays a critical role in ischemia-induced revascularization. Ischemia induced the expression and binding of c-Fos, c-Jun, JunB, FosB, and Fra2 to a noncanonical activating protein-1 (AP-1) site present in the MMP-2 promoter and decreased binding of the transcriptional repressor JunD. Ischemia also activated the expression and binding of p53 to an adjacent enhancer site (RE-1) and increased expression and binding of nuclear factor of activated T-cells-c2 to consensus sequences within the first intron. Deletion of either the 5' AP-1/RE-1 region of the promoter or substitution of the first intron abolished ischemia-induced MMP-2 transcription in vivo. Thus, AP-1 transcription factors and intronic activation by nuclear factor of activated T-cells-c2 act in concert to drive ischemia-induced MMP-2 transcription. These findings define a critical role for MMP-2 in ischemia-induced revascularization and identify both previously uncharacterized regulatory elements within the MMP-2 gene and the cognate transcription factors required for MMP-2 activation in vivo after tissue ischemia.
Figures





Similar articles
-
A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers.Biochem J. 2003 Feb 1;369(Pt 3):485-96. doi: 10.1042/BJ20020707. Biochem J. 2003. PMID: 12371906 Free PMC article.
-
Role of AP-1 and RE-1 binding sites in matrix metalloproteinase-2 transcriptional regulation in skeletal muscle atrophy.Biochem Biophys Res Commun. 2010 May 28;396(2):219-23. doi: 10.1016/j.bbrc.2010.04.067. Epub 2010 Apr 14. Biochem Biophys Res Commun. 2010. PMID: 20398633
-
Cardiac ischemia-reperfusion injury induces matrix metalloproteinase-2 expression through the AP-1 components FosB and JunB.Am J Physiol Heart Circ Physiol. 2006 Oct;291(4):H1838-46. doi: 10.1152/ajpheart.00026.2006. Epub 2006 May 12. Am J Physiol Heart Circ Physiol. 2006. PMID: 16699069
-
Matrix metalloproteinase-2 expression and promoter/enhancer activity in skeletal muscle atrophy.J Orthop Res. 2008 Mar;26(3):357-63. doi: 10.1002/jor.20513. J Orthop Res. 2008. PMID: 17960656
-
The AP-1 site and MMP gene regulation: what is all the fuss about?Matrix Biol. 1997 Mar;15(8-9):519-26. doi: 10.1016/s0945-053x(97)90026-3. Matrix Biol. 1997. PMID: 9138284 Review.
Cited by
-
FOSL2 promotes VEGF-independent angiogenesis by transcriptionnally activating Wnt5a in breast cancer-associated fibroblasts.Theranostics. 2021 Mar 5;11(10):4975-4991. doi: 10.7150/thno.55074. eCollection 2021. Theranostics. 2021. PMID: 33754039 Free PMC article.
-
Matrix metalloproteinase control of capillary morphogenesis.Crit Rev Eukaryot Gene Expr. 2008;18(3):251-78. doi: 10.1615/critreveukargeneexpr.v18.i3.30. Crit Rev Eukaryot Gene Expr. 2008. PMID: 18540825 Free PMC article. Review.
-
Repeat-induced epigenetic changes in intron 1 of the frataxin gene and its consequences in Friedreich ataxia.Nucleic Acids Res. 2007;35(10):3383-90. doi: 10.1093/nar/gkm271. Epub 2007 May 3. Nucleic Acids Res. 2007. PMID: 17478498 Free PMC article.
-
Hyperexpansion of GAA repeats affects post-initiation steps of FXN transcription in Friedreich's ataxia.Nucleic Acids Res. 2011 Oct;39(19):8366-77. doi: 10.1093/nar/gkr542. Epub 2011 Jul 10. Nucleic Acids Res. 2011. PMID: 21745819 Free PMC article.
-
Connective tissue growth factor regulates retinal neovascularization through p53 protein-dependent transactivation of the matrix metalloproteinase (MMP)-2 gene.J Biol Chem. 2012 Nov 23;287(48):40570-85. doi: 10.1074/jbc.M112.386565. Epub 2012 Oct 9. J Biol Chem. 2012. PMID: 23048035 Free PMC article.
References
-
- Tronc, F., Ziad, M., Lehoux, S., Wassef, M., Esposito, B. & Tedgui, A. (2000) Arterioscler. Thromb. Vasc. Biol. 20, e120-e126. - PubMed
-
- Takahashi, M., Fukami, S., Iwata, N., Inoue, K., Itohara, S., Itoh, H., Haraoka, J. & Saido, T. (2002) Pharmacol. Res. 46, 155-163. - PubMed
-
- Ohno-Matsui, K., Uetama, T., Yoshida, T., Hayano, M., Itoh, T., Morita, I. & Mochizuki, M. (2003) Invest. Ophthalmol. Visual Sci. 44, 5370-5375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

