Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells
- PMID: 16258069
- PMCID: PMC1274241
- DOI: 10.1073/pnas.0502598102
Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells
Abstract
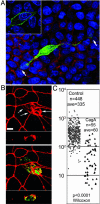
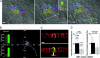
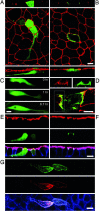
CagA is a bacterial effector protein of Helicobacter pylori that is translocated via a type IV secretion system into gastric epithelial cells. We previously described that H. pylori require CagA to disrupt the organization and assembly of apical junctions in polarized epithelial cells. In this study, we provide evidence that CagA expression is not only sufficient to disrupt the apical junctions but also perturbs epithelial differentiation. CagA-expressing cells lose apicobasal polarity and cell-cell adhesion, extend migratory pseudopodia, and degrade basement membranes, acquiring an invasive phenotype. Expression of the CagA C-terminal domain, which contains the tyrosine phosphorylated EPIYA motifs, induces pseudopodial activity but is not sufficient to induce cell migration. Conversely, the N terminus targets CagA to the cell-cell junctions. Neither domain is sufficient to disrupt cell adhesion or cell polarity, but coexpressed in trans, the N terminus determines the localization of both polypeptides. We show that CagA induces a morphogenetic program in polarized Madin-Darby canine kidney cells resembling an epithelial-to-mesenchymal transition. We propose that altered cell-cell and cell matrix interactions may serve as an early event in H. pylori-induced carcinogenesis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

