Protocol for: Sheffield Obesity Trial (SHOT): a randomised controlled trial of exercise therapy and mental health outcomes in obese adolescents [ISRCNT83888112]
- PMID: 16259624
- PMCID: PMC1310532
- DOI: 10.1186/1471-2458-5-113
Protocol for: Sheffield Obesity Trial (SHOT): a randomised controlled trial of exercise therapy and mental health outcomes in obese adolescents [ISRCNT83888112]
Abstract
Background: While obesity is known to have many physiological consequences, the psychopathology of this condition has not featured prominently in the literature. Cross-sectional studies have indicated that obese children have increased odds of experiencing poor quality of life and mental health. However, very limited trial evidence has examined the efficacy of exercise therapy for enhancing mental health outcomes in obese children, and the Sheffield Obesity Trial (SHOT) will provide evidence of the efficacy of supervised exercise therapy in obese young people aged 11-16 years versus usual care and an attention-control intervention.
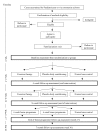
Method/design: SHOT is a randomised controlled trial where obese young people are randomised to receive; (1) exercise therapy, (2) attention-control intervention (involving body-conditioning exercises and games that do not involve aerobic activity), or (3) usual care. The exercise therapy and attention-control sessions will take place three times per week for eight weeks and a six-week home programme will follow this. Ninety adolescents aged between 11-16 years referred from a children's hospital for evaluation of obesity or via community advertisements will need to complete the study. Participants will be recruited according to the following criteria: (1) clinically obese and aged 11-16 years (Body Mass Index Centile > 98th UK standard) (2) no medical condition that would restrict ability to be active three times per week for eight weeks and (3) not diagnosed with insulin dependent diabetes or receiving oral steroids. Assessments of outcomes will take place at baseline, as well as four (intervention midpoint) and eight weeks (end of intervention) from baseline. Participants will be reassessed on outcome measures five and seven months from baseline. The primary endpoint is physical self-perceptions. Secondary outcomes include physical activity, self-perceptions, depression, affect, aerobic fitness and BMI.
Figures
References
-
- World Health Organisation . Obesity: preventing and managing the global epidemic. Geneva. WHO; 1987. [WHO Technical Report Series: No 894] - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical