Snm1-deficient mice exhibit accelerated tumorigenesis and susceptibility to infection
- PMID: 16260620
- PMCID: PMC1280277
- DOI: 10.1128/MCB.25.22.10071-10078.2005
Snm1-deficient mice exhibit accelerated tumorigenesis and susceptibility to infection
Abstract
The eukaryotic SNM1 gene family has been implicated in a number of cellular pathways, including repair of DNA interstrand cross-links, involvement in VDJ recombination, repair of DNA double-strand breaks, and participation in cell cycle checkpoint pathways. In particular, mammalian SNM1 has been shown to be required in a mitotic checkpoint that causes arrest of cells in prophase prior to chromosome condensation in response to spindle poisons. Here, we report on the phenotype of a knockout of Snm1 in the mouse. Snm1-/- mice are viable and fertile but exhibit a complex phenotype. Both homozygous and heterozygous mice show a decline in survival compared to wild-type littermates. In homozygous mutant males, this reduction in survival is principally due to bacterial infections in the preputial and mandibular glands and to a lesser extent to tumorigenesis, while in homozygous and heterozygous females, it is due almost solely to tumorigenesis. The high incidence of bacterial infections in the homozygous mutant males suggests an immune dysfunction; however, examinations of T- and B-cell development and immunoglobulin class switching did not reveal a defect in these pathways. Crossing of Snm1 mutant mice with a Trp53 null mutant resulted in an increase in mortality and a restriction of the tumor type to lymphomas, particularly those of the thymus. Taken together, these findings demonstrate that Snm1 is a tumor suppressor in mice that in addition has a role in immunity.
Figures
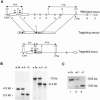
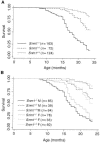

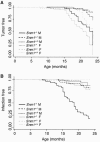


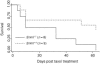
References
-
- Bassing, C. H., H. Suh, D. O. Ferguson, K. F. Chua, J. Manis, M. Eckersdorff, M. Gleason, R. Bronson, C. Lee, and F. W. Alt. 2003. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell 114:359-370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
