Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein
- PMID: 16260631
- PMCID: PMC1280279
- DOI: 10.1128/MCB.25.22.10190-10201.2005
Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein
Abstract
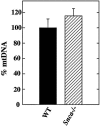
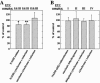
The presynaptic protein alpha-synuclein, implicated in Parkinson disease (PD), binds phospholipids and has a role in brain fatty acid (FA) metabolism. In mice lacking alpha-synuclein (Snca-/-), total brain steady-state mass of the mitochondria-specific phospholipid, cardiolipin, is reduced 22% and its acyl side chains show a 51% increase in saturated FAs and a 25% reduction in essential n-6, but not n-3, polyunsaturated FAs. Additionally, 23% reduction in phosphatidylglycerol content, the immediate biosynthetic precursor of cardiolipin, was observed without alterations in the content of other brain phospholipids. Consistent with these changes, more ordered lipid head group and acyl chain packing with enhanced rotational motion of diphenylhexatriene (DPH) about its long axis were demonstrated in time-resolved DPH fluorescence lifetime experiments. These abnormalities in mitochondrial membrane properties were associated with a 15% reduction in linked complex I/III activity of the electron transport chain, without reductions in mitochondrial number, complex II/III activity, or individual complex I, II, III, or IV activity. Reduced complex I activity is thought to be a critical factor in the development of PD. Thus, altered membrane composition and structure and impaired complex I/III function in Snca-/- brain suggest a relationship between alpha-synuclein's role in brain lipid metabolism, mitochondrial function, and PD.
Figures
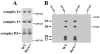
References
-
- Abeliovich, A., Y. Schmitz, I. Farinas, D. Choi-Lundberg, W. H. Ho, P. E. Castillo, N. Shinsky, J. M. Verdugo, M. Armanini, A. Ryan, M. Hynes, H. Phillips, D. Sulzer, and A. Rosenthal. 2000. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239-252. - PubMed
-
- Akesson, B., J. Elovsson, and G. Arvidsson. 1970. Initial incorporation into rat liver glycerolipids of intraportally injected [3H] glycerol. Biochim. Biophys. Acta 210:15-27. - PubMed
-
- Barcelo-Coblijn, G., K. Kitajka, L. G. Puskas, E. Hogyes, A. Zvara, L. Hackler, Jr., and T. Farkas. 2003. Gene expression and molecular composition of phospholipids in rat brain in relation to dietary n-6 to n-3 fatty acid ratio. Biochim. Biophys. Acta 1632:72-79. - PubMed
-
- Barth, P. G., F. Valianpour, V. M. Bowen, J. Lam, M. Duran, F. M. Vaz, and R. J. A. Wanders. 2004. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am. J. Med. Genet. 126A:349-354. - PubMed
-
- Birch-Machin, M. A., and D. M. Turnbull. 2001. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Mitochondria 65:97-117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
