IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism
- PMID: 16260731
- PMCID: PMC1283464
- DOI: 10.1073/pnas.0508237102
IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism
Abstract
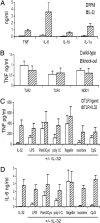
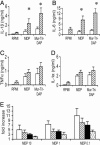
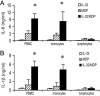
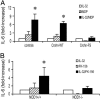
The activation of innate immunity requires the amplification of signals induced by pattern-recognition receptors for bacterial products. We have investigated the role of the newly described cytokine IL-32 in the amplification of cytokine production induced by the two most clinically relevant families of microbial receptors, the cell-surface Toll-like receptors (TLRs) and the intracellular nuclear oligomerization domain (NOD) receptor family. IL-32 synergized with the NOD1- and NOD2-specific muropeptides of peptidoglycans for the release of IL-1beta and IL-6 (a 3- to 10-fold increase). In contrast, IL-32 did not influence the cytokine production induced via TLRs. The synergistic effect of IL-32 and synthetic muramyl dipeptide (MDP) on cytokine production was absent in the cells of patients with Crohn's disease bearing the NOD2 frameshift mutation 3020insC, demonstrating that the IL-32/MDP synergism depends on NOD2. This in vitro synergism between IL-32 and NOD2 ligands was consistent with a marked constitutive expression of IL-32 in human colon epithelial tissue. In addition, the potentiating effect of IL-32 on the cytokine production induced by the synthetic muropeptide FK-156 was absent in NOD1-deficient macrophages, supporting the interaction between IL-32 and NOD1 pathways. When specific caspase inhibitors were used, the synergism between IL-32 and MDP/NOD2 depended on the activation of caspase 1. Only additive effects of IL-32 and muropeptides were observed for TNF-alpha production. The modulation of intracellular NOD2 pathways by IL-32, but not cell-surface TLRs, and the marked expression of IL-32 in colon mucosa suggest a role of IL-32 in the pathogenesis of Crohn's disease.
Figures

References
-
- Kim, S. H., Han, S. Y., Azam, T., Yoon, D. Y. & Dinarello, C. A. (2005) Immunity 22, 131-142. - PubMed
-
- Medzhitov, R. (2001) Nat. Rev. Immunol. 1, 135-145. - PubMed
-
- Beutler, B. (2004) Nature 430, 257-263. - PubMed
-
- Kapsenberg, M. (2003) Nat. Rev. Immunol. 3, 984-993. - PubMed
-
- Inohara, N., Ogura, Y. & Nunez, G. (2002) Curr. Opin. Microbiol. 5, 76-80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

