Brain lactate kinetics: Modeling evidence for neuronal lactate uptake upon activation
- PMID: 16260743
- PMCID: PMC1297516
- DOI: 10.1073/pnas.0505427102
Brain lactate kinetics: Modeling evidence for neuronal lactate uptake upon activation
Abstract
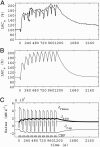
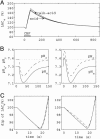
A critical issue in brain energy metabolism is whether lactate produced within the brain by astrocytes is taken up and metabolized by neurons upon activation. Although there is ample evidence that neurons can efficiently use lactate as an energy substrate, at least in vitro, few experimental data exist to indicate that it is indeed the case in vivo. To address this question, we used a modeling approach to determine which mechanisms are necessary to explain typical brain lactate kinetics observed upon activation. On the basis of a previously validated model that takes into account the compartmentalization of energy metabolism, we developed a mathematical model of brain lactate kinetics, which was applied to published data describing the changes in extracellular lactate levels upon activation. Results show that the initial dip in the extracellular lactate concentration observed at the onset of stimulation can only be satisfactorily explained by a rapid uptake within an intraparenchymal cellular compartment. In contrast, neither blood flow increase, nor extracellular pH variation can be major causes of the lactate initial dip, whereas tissue lactate diffusion only tends to reduce its amplitude. The kinetic properties of monocarboxylate transporter isoforms strongly suggest that neurons represent the most likely compartment for activation-induced lactate uptake and that neuronal lactate utilization occurring early after activation onset is responsible for the initial dip in brain lactate levels observed in both animals and humans.
Figures


References
-
- Siesjö, B. K. (1978) Brain Energy Metabolism (Wiley, New York).
-
- Sappey-Marinier, D., Calabrese, G., Fein, G., Hugg, J. W., Biggins, C. & Weiner, M. W. (1992) J. Cereb. Blood Flow Metab. 12, 584-592. - PubMed
-
- Frahm, J., Krüger, G., Merboldt, K.-D. & Kleinschmidt, A. (1996) Magn. Reson. Med. 35, 143-148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

