High-efficiency transformation of Plasmodium falciparum by the lepidopteran transposable element piggyBac
- PMID: 16260745
- PMCID: PMC1275597
- DOI: 10.1073/pnas.0504679102
High-efficiency transformation of Plasmodium falciparum by the lepidopteran transposable element piggyBac
Abstract
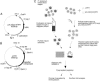
Functional analysis of the Plasmodium falciparum genome is restricted because of the limited ability to genetically manipulate this important human pathogen. We have developed an efficient transposon-mediated insertional mutagenesis method much needed for high-throughput functional genomics of malaria parasites. A drug-selectable marker, human dihydrofolate reductase, added to the lepidopteran transposon piggyBac, transformed parasites by integration into the P. falciparum genome in the presence of a transposase-expressing helper plasmid. Multiple integrations occurred at the expected TTAA target sites throughout the genome of the parasite. We were able to transform P. falciparum with this piggyBac element at high frequencies, in the range of 10(-3), and obtain stable clones of insertional mutants in a few weeks instead of 6-12 months. Our results show that the piggyBac transposition system can be used as an efficient, random integration tool needed for large-scale, whole-genome mutagenesis of malaria parasites. The availability of such an adaptable genetic tool opens the way for much needed forward genetic approaches to study this lethal human parasite.
Figures
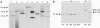

References
-
- Greenwood, B. & Mutabingwa, T. (2002) Nature 415, 670-672. - PubMed
-
- Maitland, K., Makanga, M. & Williams, T. N. (2004) Curr. Opin. Infect. Dis. 17, 405-412. - PubMed
-
- Breman, J. G., Alilio, M. S. & Mills, A. (2004) Am. J. Trop. Med. Hyg. 71, 1-15. - PubMed
-
- Hartl, D. L. (2004) Nat. Rev. Microbiol. 2, 15-22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

