Long-range protein electron transfer observed at the single-molecule level: In situ mapping of redox-gated tunneling resonance
- PMID: 16260751
- PMCID: PMC1275599
- DOI: 10.1073/pnas.0508257102
Long-range protein electron transfer observed at the single-molecule level: In situ mapping of redox-gated tunneling resonance
Abstract
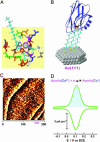
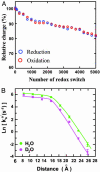
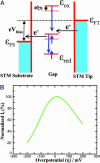
A biomimetic long-range electron transfer (ET) system consisting of the blue copper protein azurin, a tunneling barrier bridge, and a gold single-crystal electrode was designed on the basis of molecular wiring self-assembly principles. This system is sufficiently stable and sensitive in a quasi-biological environment, suitable for detailed observations of long-range protein interfacial ET at the nanoscale and single-molecule levels. Because azurin is located at clearly identifiable fixed sites in well controlled orientation, the ET configuration parallels biological ET. The ET is nonadiabatic, and the rate constants display tunneling features with distance-decay factors of 0.83 and 0.91 A(-1) in H(2)O and D(2)O, respectively. Redox-gated tunneling resonance is observed in situ at the single-molecule level by using electrochemical scanning tunneling microscopy, exhibiting an asymmetric dependence on the redox potential. Maximum resonance appears around the equilibrium redox potential of azurin with an on/off current ratio of approximately 9. Simulation analyses, based on a two-step interfacial ET model for the scanning tunneling microscopy redox process, were performed and provide quantitative information for rational understanding of the ET mechanism.
Figures


References
-
- Marcus, R. & Sutin, A. N. (1985) Biochim. Biophys. Acta 811, 265–322.
-
- Barbara, P. F., Meyer, T. J. & Ratner, M. A. (1996) J. Phys. Chem. 100, 13148–13168.
-
- Jortner, J. & Ratner, M. A., eds. (1997) Molecular Electronics (Blackwell, Oxford).
-
- Jortner, J. & Bixon, M., eds. (1998) Electron Transfer from Isolated Molecules to Biomolecules: Advances in Chemical Physics (Wiley, New York), Vols. 106 and 107.
-
- Kuznetrov, A. M. & Ulstrup, J. (1999) Electron Transfer in Chemistry and Biology: An Introduction to the Theory (Wiley, Chichester, U.K.).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

