A spatiotemporal white noise analysis of photoreceptor responses to UV and green light in the dragonfly median ocellus
- PMID: 16260838
- PMCID: PMC2266605
- DOI: 10.1085/jgp.200509319
A spatiotemporal white noise analysis of photoreceptor responses to UV and green light in the dragonfly median ocellus
Abstract
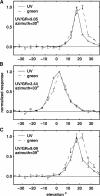
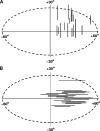
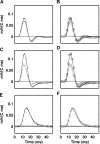
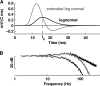
Adult dragonflies augment their compound eyes with three simple eyes known as the dorsal ocelli. While the ocellar system is known to mediate stabilizing head reflexes during flight, the ability of the ocellar retina to dynamically resolve the environment is unknown. For the first time, we directly measured the angular sensitivities of the photoreceptors of the dragonfly median (middle) ocellus. We performed a second-order Wiener Kernel analysis of intracellular recordings of light-adapted photoreceptors. These were stimulated with one-dimensional horizontal or vertical patterns of concurrent UV and green light with different contrast levels and at different ambient temperatures. The photoreceptors were found to have anisotropic receptive fields with vertical and horizontal acceptance angles of 15 degrees and 28 degrees, respectively. The first-order (linear) temporal kernels contained significant undershoots whose amplitudes are invariant under changes in the contrast of the stimulus but significantly reduced at higher temperatures. The second-order kernels showed evidence of two distinct nonlinear components: a fast acting self-facilitation, which is dominant in the UV, followed by delayed self- and cross-inhibition of UV and green light responses. No facilitatory interactions between the UV and green light were found, indicating that facilitation of the green and UV responses occurs in isolated compartments. Inhibition between UV and green stimuli was present, indicating that inhibition occurs at a common point in the UV and green response pathways. We present a nonlinear cascade model (NLN) with initial stages consisting of separate UV and green pathways. Each pathway contains a fast facilitating nonlinearity coupled to a linear response. The linear response is described by an extended log-normal model, accounting for the phasic component. The final nonlinearity is composed of self-inhibition in the UV and green pathways and inhibition between these pathways. The model can largely predict the response of the photoreceptors to UV and green light.
Figures






References
-
- Anderson, J.C., and S.B. Laughlin. 2000. Photoreceptor performance and the coordination of achromatic and chromatic inputs in the fly visual system. Vision Res. 40:13–31. - PubMed
-
- Corbet, P.S. 1999. Dragonflies: behaviour and ecology of Odonata. Harley Books, Martins, Essex, UK. 829 pp.

