Rescue of heterochromatin organization in Hutchinson-Gilford progeria by drug treatment
- PMID: 16261260
- PMCID: PMC2773834
- DOI: 10.1007/s00018-005-5318-6
Rescue of heterochromatin organization in Hutchinson-Gilford progeria by drug treatment
Abstract
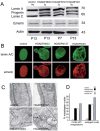
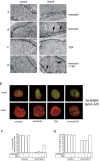
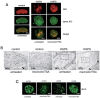
Hutchinson-Gilford progeria (HGPS) is a premature aging syndrome associated with LMNA mutations. Progeria cells bearing the G608G LMNA mutation are characterized by accumulation of a mutated lamin A precursor (progerin), nuclear dysmorphism and chromatin disorganization. In cultured HGPS fibroblasts, we found worsening of the cellular phenotype with patient age, mainly consisting of increased nuclear-shape abnormalities, progerin accumulation and heterochromatin loss. Moreover, transcript distribution was altered in HGPS nuclei, as determined by different techniques. In the attempt to improve the cellular phenotype, we applied treatment with drugs either affecting protein farnesylation or chromatin arrangement. Our results show that the combined treatment with mevinolin and the histone deacetylase inhibitor trichostatin A dramatically lowers progerin levels, leading to rescue of heterochromatin organization and reorganization of transcripts in HGPS fibroblasts. These results suggest that morpho-functional defects of HGPS nuclei are directly related to progerin accumulation and can be rectified by drug treatment.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

