Regulation of cysteine dioxygenase degradation is mediated by intracellular cysteine levels and the ubiquitin-26 S proteasome system in the living rat
- PMID: 16262602
- PMCID: PMC1386025
- DOI: 10.1042/BJ20051510
Regulation of cysteine dioxygenase degradation is mediated by intracellular cysteine levels and the ubiquitin-26 S proteasome system in the living rat
Abstract
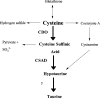
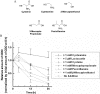
Mammalian metabolism of ingested cysteine is conducted principally within the liver. The liver tightly regulates its intracellular cysteine pool to keep levels high enough to meet the many catabolic and anabolic pathways for which cysteine is needed, but low enough to prevent toxicity. One of the enzymes the liver uses to regulate cysteine levels is CDO (cysteine dioxygenase). Catalysing the irreversible oxidation of cysteine, CDO protein is up-regulated in the liver in response to the dietary intake of cysteine. In the present study, we have evaluated the contribution of the ubiquitin-26 S proteasome pathway to the diet-induced changes in CDO half-life. In the living rat, inhibition of the proteasome with PS1 (proteasome inhibitor 1) dramatically stabilized CDO in the liver under dietary conditions that normally favour its degradation. Ubiquitinated CDO intermediates were also seen to accumulate in the liver. Metabolic analyses showed that PS1 had a significant effect on sulphoxidation flux secondary to the stabilization of CDO but no significant effect on the intracellular cysteine pool. Finally, by a combination of in vitro hepatocyte culture and in vivo whole animal studies, we were able to attribute the changes in CDO stability specifically to cysteine rather than the metabolite 2-mercaptoethylamine (cysteamine). The present study represents the first demonstration of regulated ubiquitination and degradation of a protein in a living mammal, inhibition of which had dramatic effects on cysteine catabolism.
Figures


References
-
- Andine P., Orwar O., Jacobson I., Sandberg M., Hagberg H. Extracellular acidic sulfur-containing amino acids and gamma-glutamyl peptides in global ischemia: postischemic recovery of neuronal activity is paralleled by a tetrodotoxin-sensitive increase in cysteine sulfinate in the CA1 of the rat hippocampus. J. Neurochem. 1991;57:230–236. - PubMed
-
- Lehmann A. Alterations in hippocampal extracellular amino acids and purine catabolites during limbic seizures induced by folate injections into the rabbit amygdala. Neuroscience. 1987;22:573–578. - PubMed
-
- Lehmann A., Hagberg H., Orwar O., Sandberg M. Cysteine sulphinate and cysteate: mediators of cysteine toxicity in the neonatal rat brain? Eur. J. Neurosci. 1993;5:1398–1412. - PubMed
-
- Bradley H., Gough A., Sokhi R. S., Hassell A., Waring R., Emery P. Sulfate metabolism is abnormal in patients with rheumatoid arthritis. Confirmation by in vivo biochemical findings. J. Rheumatol. 1994;21:1192–1196. - PubMed
-
- Heafield M. T., Fearn S., Steventon G. B., Waring R. H., Williams A. C., Sturman S. G. Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson's and Alzheimer's disease. Neurosci. Lett. 1990;110:216–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

