Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells
- PMID: 16262603
- PMCID: PMC1386030
- DOI: 10.1042/BJ20051298
Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells
Abstract
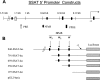
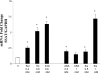
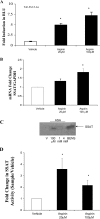
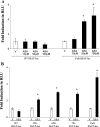
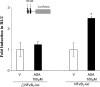
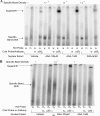
Epidemiological, experimental and clinical results suggest that aspirin and other NSAIDs (non-steroidal anti-inflammatory drugs) inhibit the development of colon cancer. It has been shown that the NSAID sulindac induces apoptosis and suppresses carcinogenesis, in part, by a mechanism leading to the transcriptional activation of the gene encoding SSAT (spermidine/spermine N1-acetyltransferase), a rate-limiting enzyme in polyamine catabolism. In the present study, we show that a variety of NSAIDs, including aspirin, sulindac, ibuprofen and indomethacin, can induce SSAT gene expression in Caco-2 cells. Aspirin, at physiological concentrations, can induce SSAT mRNA via transcriptional initiation mechanisms. This induction leads to increased SSAT protein levels and enzyme activity. Promoter deletion analysis of the 5' SSAT promoter-flanking region led to the identification of two NF-kappaB (nuclear factor kappaB) response elements. Electrophoretic mobility-shift assays showed binding of NF-kappaB complexes at these sequences after aspirin treatment. Aspirin treatment led to the activation of NF-kappaB signalling and increased binding at these NF-kappaB sites in the SSAT promoter, hence providing a potential mechanism for the induction of SSAT by aspirin in these cells. Aspirin-induced SSAT ultimately leads to a decrease in cellular polyamine content, which has been associated with decreased carcinogenesis. These results suggest that activation of SSAT by aspirin and different NSAIDs may be a common property of NSAIDs that plays an important role in their chemopreventive actions in colorectal cancer.
Figures
References
-
- Vane J. R., Flower R. J., Botting R. M. History of aspirin and its mechanism of action. Stroke. 1990;21:IV12–IV23. - PubMed
-
- Piazza G. A., Alberts D. S., Hixson L. J., Paranka N. S., Li H., Finn T., Bogert C., Guillen J. M., Brendel K., Gross P. H., et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57:2909–2915. - PubMed
-
- Shiff S. J., Koutsos M. I., Qiao L., Rigas B. Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: effects on cell cycle and apoptosis. Exp. Cell Res. 1996;222:179–188. - PubMed
-
- Thompson W. J., Piazza G. A., Li H., Liu L., Fetter J., Zhu B., Sperl G., Ahnen D., Pamukcu R. Exisulind induction of apoptosis involves guanosine 3′,5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000;60:3338–3342. - PubMed
-
- Turchanowa L., Dauletbaev N., Milovic V., Stein J. Nonsteroidal anti-inflammatory drugs stimulate spermidine/spermine acetyltransferase and deplete polyamine content in colon cancer cells. Eur. J. Clin. Invest. 2001;31:887–893. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

