Domain II plays a crucial role in the function of ribosome recycling factor
- PMID: 16262604
- PMCID: PMC1360730
- DOI: 10.1042/BJ20050780
Domain II plays a crucial role in the function of ribosome recycling factor
Abstract
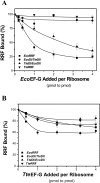
RRF (ribosome recycling factor) consists of two domains, and in concert with EF-G (elongation factor-G), triggers dissociation of the post-termination ribosomal complex. However, the function of the individual domains of RRF remains unclear. To clarify this, two RRF chimaeras, EcoDI/TteDII and TteDI/EcoDII, were created by domain swaps between the proteins from Escherichia coli and Thermoanaerobacter tengcongensis. The ribosome recycling activity of the RRF chimaeras was compared with their wild-type RRFs by using in vivo and in vitro activity assays. Like wild-type TteRRF (T. tengcongensis RRF), the EcoDI/TteDII chimaera is non-functional in E. coli, but both wild-type TteRRF, and EcoDI/TteDII can be activated by coexpression of T. tengcongensis EF-G in E. coli. By contrast, like wild-type E. coli RRF (EcoRRF), TteDI/EcoDII is fully functional in E. coli. These findings suggest that domain II of RRF plays a crucial role in the concerted action of RRF and EF-G for the post-termination complex disassembly, and the specific interaction between RRF and EF-G on ribosomes mainly depends on the interaction between domain II of RRF and EF-G. This study provides direct genetic and biochemical evidence for the function of the individual domains of RRF.
Figures



Similar articles
-
Expression in Escherichia coli, purification and characterization of Thermoanaerobacter tengcongensis ribosome recycling factor.J Biochem. 2005 Jul;138(1):89-94. doi: 10.1093/jb/mvi102. J Biochem. 2005. PMID: 16046452
-
Heterologous expression of Aquifex aeolicus ribosome recycling factor in Escherichia coli is dominant lethal by forming a complex that lacks functional co-ordination for ribosome disassembly.Mol Microbiol. 2005 Jan;55(1):150-61. doi: 10.1111/j.1365-2958.2004.04387.x. Mol Microbiol. 2005. PMID: 15612924
-
Specific interaction between the ribosome recycling factor and the elongation factor G from Mycobacterium tuberculosis mediates peptidyl-tRNA release and ribosome recycling in Escherichia coli.EMBO J. 2001 Jun 1;20(11):2977-86. doi: 10.1093/emboj/20.11.2977. EMBO J. 2001. PMID: 11387230 Free PMC article.
-
[Ribosome recycling revisited].Mol Biol (Mosk). 2006 Jul-Aug;40(4):742-50. Mol Biol (Mosk). 2006. PMID: 16913233 Review. Russian.
-
Dual functions of ribosome recycling factor in protein biosynthesis: disassembling the termination complex and preventing translational errors.Biochimie. 1996;78(11-12):959-69. doi: 10.1016/s0300-9084(97)86718-1. Biochimie. 1996. PMID: 9150873 Review.
Cited by
-
Mechanisms of ribosome recycling in bacteria and mitochondria: a structural perspective.RNA Biol. 2022;19(1):662-677. doi: 10.1080/15476286.2022.2067712. Epub 2021 Dec 31. RNA Biol. 2022. PMID: 35485608 Free PMC article. Review.
-
Purification, crystallization and preliminary crystallographic analysis of a ribosome-recycling factor from Thermoanaerobacter tengcongensis (TteRRF).Acta Crystallogr F Struct Biol Commun. 2014 May;70(Pt 5):588-91. doi: 10.1107/S2053230X1400507X. Epub 2014 Apr 15. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 24817715 Free PMC article.
-
Structural insights into initial and intermediate steps of the ribosome-recycling process.EMBO J. 2012 Apr 4;31(7):1836-46. doi: 10.1038/emboj.2012.22. Epub 2012 Mar 2. EMBO J. 2012. PMID: 22388519 Free PMC article.
-
Structural Insights into ribosome recycling factor interactions with the 70S ribosome.J Mol Biol. 2008 Mar 7;376(5):1334-47. doi: 10.1016/j.jmb.2007.12.048. Epub 2008 Jan 3. J Mol Biol. 2008. PMID: 18234219 Free PMC article.
-
Mechanistic insights into the alternative ribosome recycling by HflXr.Nucleic Acids Res. 2024 Apr 24;52(7):4053-4066. doi: 10.1093/nar/gkae128. Nucleic Acids Res. 2024. PMID: 38407413 Free PMC article.
References
-
- Zavialov A. V., Buckingham R. H., Ehrenberg M. A posttermination ribosomal complex is the guanine nucleotide exchange factor for peptide release factor RF3. Cell. 2001;107:115–124. - PubMed
-
- Zavialov A. V., Mora L., Buckingham R. H., Ehrenberg M. Release of peptide promoted by the GGQ motif of class 1 release factors regulates the GTPase activity of RF3. Mol. Cell. 2002;10:789–798. - PubMed
-
- Janosi L., Hara H., Zhang S., Kaji A. Ribosome recycling by ribosome recycling factor (RRF)- an important but overlooked step of protein synthesis. Adv. Biophys. 1996;32:121–201. - PubMed
-
- Janosi L., Ricker R., Kaji A. Dual functions of ribosome recycling factor in protein biosynthesis: disassembling the termination complex and preventing translational errors. Biochimie. 1996;78:959–969. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

