Organotins disrupt the 11beta-hydroxysteroid dehydrogenase type 2-dependent local inactivation of glucocorticoids
- PMID: 16263518
- PMCID: PMC1310925
- DOI: 10.1289/ehp.8209
Organotins disrupt the 11beta-hydroxysteroid dehydrogenase type 2-dependent local inactivation of glucocorticoids
Abstract
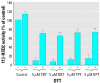
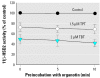
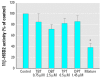
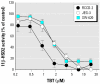
Organotins, important environmental pollutants widely used in agricultural and industrial applications, accumulate in the food chain and induce imposex in several marine species as well as neurotoxic and immunotoxic effects in higher animals. Reduced birth weight and thymus involution, observed upon exposure to organotins, can also be caused by excessive glucocorticoid levels. We now demonstrate that organotins efficiently inhibit 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2), converting active 11beta-hydroxyglucocorticoids into inactive 11-ketoglucocorticoids, but not 11beta-HSD1, which catalyzes the reverse reaction. Di- and tributyltin as well as di- and triphenyltin inhibited recombinant and endogenous 11beta-HSD2 in lysates and intact cells with IC50 values between 500 nM and 3 microM. Dithiothreitol protected 11beta-HSD2 from organotin-dependent inhibition, indicating that organotins act by binding to one or more cysteines. Mutational analysis and 3-D structural modeling revealed several important interactions of cysteines in 11beta-HSD2. Cys90, Cys228, and Cys264 were essential for enzymatic stability and catalytic activity, suggesting that disruption of such interactions by organotins leads to inhibition of 11beta-HSD2. Enhanced glucocorticoid concentrations due to disruption of 11beta-HSD2 function may contribute to the observed organotin-dependent toxicity in some glucocorticoid-sensitive tissues such as thymus and placenta.
Figures



References
-
- Adeeko A, Li D, Forsyth DS, Casey V, Cooke GM, Barthelemy J, et al. Effects of in utero tributyltin chloride exposure in the rat on pregnancy outcome. Toxicol Sci. 2003;74:407–415. - PubMed
-
- Arnold P, Tam S, Yan L, Baker ME, Frey FJ, Odermatt A. Glutamate-115 renders specificity of human 11β-hydroxysteroid dehydrogenase type 2 for the cofactor NAD(+) Mol Cell Endocrinol. 2003;201:177–187. - PubMed
-
- Atanasov AG, Nashev LG, Schweizer RA, Frick C, Odermatt A. Hexose-6-phosphate dehydrogenase determines the reaction direction of 11β-hydroxysteroid dehydrogenase type 1 as an oxoreductase. FEBS Lett. 2004;571:129–133. - PubMed
-
- Atanasov AG, Tam S, Rocken JM, Baker ME, Odermatt A. Inhibition of 11β-hydroxysteroid dehydrogenase type 2 by dithiocarbamates. Biochem Biophys Res Commun. 2003;308:257–262. - PubMed
-
- Baker ME. Evolutionary analysis of 11β-hydroxysteroid dehydrogenase-type 1, -type 2, -type 3 and 17β-hydroxysteroid dehydrogenase-type 2 in fish. FEBS Lett. 2004;574:167–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

