The PAS/LOV protein VIVID supports a rapidly dampened daytime oscillator that facilitates entrainment of the Neurospora circadian clock
- PMID: 16264193
- PMCID: PMC1276733
- DOI: 10.1101/gad.349305
The PAS/LOV protein VIVID supports a rapidly dampened daytime oscillator that facilitates entrainment of the Neurospora circadian clock
Abstract
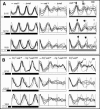
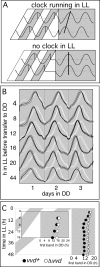
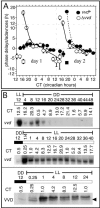
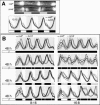
A light-entrainable circadian clock controls development and physiology in Neurospora crassa. Existing simple models for resetting based on light pulses (so-called nonparametric entrainment) predict that constant light should quickly send the clock to an arrhythmic state; however, such a clock would be of little use to an organism in changing photoperiods in the wild, and we confirm that true, albeit dampened, rhythmicity can be observed in extended light. This rhythmicity requires the PAS/LOV protein VIVID (VVD) that acts, in the light, to facilitate expression of an oscillator that is related to, but distinguishable from, the classic FREQUENCY/WHITE-COLLAR complex (FRQ/WCC)-based oscillator that runs in darkness. VVD prevents light resetting of the clock at dawn but, by influencing frq RNA turnover, promotes resetting at dusk, thereby allowing the clock to run through the dawn transition and take its phase cues from dusk. Consistent with this, loss of VVD yields a clock whose performance follows the simple predictions of earlier models, and overexpression of VVD restores rhythmicity in the light and sensitivity of phase to the duration of the photoperiod.
Figures


Similar articles
-
VIVID interacts with the WHITE COLLAR complex and FREQUENCY-interacting RNA helicase to alter light and clock responses in Neurospora.Proc Natl Acad Sci U S A. 2010 Sep 21;107(38):16709-14. doi: 10.1073/pnas.1009474107. Epub 2010 Aug 31. Proc Natl Acad Sci U S A. 2010. PMID: 20807745 Free PMC article.
-
The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating, and regulates clock resetting.Cell. 2001 Feb 9;104(3):453-64. doi: 10.1016/s0092-8674(01)00232-x. Cell. 2001. PMID: 11239402
-
Circadian clock genes frequency and white collar-1 are not essential for entrainment to temperature cycles in Neurospora crassa.Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4469-74. doi: 10.1073/pnas.0510404103. Epub 2006 Mar 14. Proc Natl Acad Sci U S A. 2006. PMID: 16537415 Free PMC article.
-
The molecular workings of the Neurospora biological clock.Novartis Found Symp. 2003;253:184-98; discussion 102-9, 198-202, 281-4. Novartis Found Symp. 2003. PMID: 14712922 Review.
-
Light input and processing in the circadian clock of Neurospora.FEBS Lett. 2011 May 20;585(10):1467-73. doi: 10.1016/j.febslet.2011.03.050. Epub 2011 Mar 29. FEBS Lett. 2011. PMID: 21453703 Review.
Cited by
-
Temperature-sensitive and circadian oscillators of Neurospora crassa share components.Genetics. 2012 May;191(1):119-31. doi: 10.1534/genetics.111.137976. Epub 2012 Feb 23. Genetics. 2012. PMID: 22367035 Free PMC article.
-
Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity.PLoS Genet. 2015 May 15;11(5):e1005215. doi: 10.1371/journal.pgen.1005215. eCollection 2015 May. PLoS Genet. 2015. PMID: 25978382 Free PMC article.
-
Conidiation rhythm and light entrainment in superoxide dismutase mutant in Neurospora crassa.Mol Genet Genomics. 2008 Feb;279(2):193-202. doi: 10.1007/s00438-007-0308-z. Epub 2007 Dec 13. Mol Genet Genomics. 2008. PMID: 18084778
-
Natural Variation of the Circadian Clock in Neurospora.Adv Genet. 2017;99:1-37. doi: 10.1016/bs.adgen.2017.09.001. Epub 2017 Oct 12. Adv Genet. 2017. PMID: 29050553 Free PMC article. Review.
-
Circadian rhythms in Neurospora crassa and other filamentous fungi.Eukaryot Cell. 2006 Aug;5(8):1184-93. doi: 10.1128/EC.00133-06. Eukaryot Cell. 2006. PMID: 16896204 Free PMC article. Review. No abstract available.
References
-
- Aronson B.D., Johnson, K.A., Loros, J.J., and Dunlap, J.C. 1994b. Negative feedback defining a circadian clock: Auto-regulation of the clock gene frequency. Science 263: 1578-1584. - PubMed
-
- Ashmore L.J. and Sehgal, A. 2003. A fly's eye view of circadian entrainment. J. Biol. Rhythms 18: 206-216. - PubMed
-
- Cermakian N. and Sassone-Corsi, P. 2000. Multilevel regulation of the circadian clock. Nat. Rev. Mol. Cell. Biol. 1: 59-67. - PubMed
-
- Chang B. and Nakashima, H. 1997. Effects of light-dark cycles on the circadian conidiation rhythm in Neurospora crassa. J. Plant. Res. 110: 449-453.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
