Bile-salt-stimulated lipase and mucins from milk of 'secretor' mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands
- PMID: 16266293
- PMCID: PMC1360715
- DOI: 10.1042/BJ20050898
Bile-salt-stimulated lipase and mucins from milk of 'secretor' mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands
Abstract
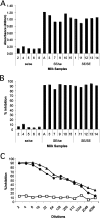
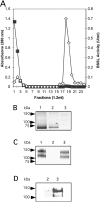
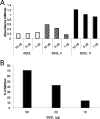
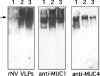
Breast-feeding-associated protection against calicivirus diarrhoea is associated with the presence of high levels of 2-linked oligosaccharides in mother's milk, and human calicivirus strains including the NV (Norwalk virus) use gut 2-linked fucosylated glycans as receptors, suggesting the presence of decoy receptors in milk. Our aim was to analyse the ability of human milk to inhibit the attachment of rNV VLPs (recombinant NV-like particles) to their carbohydrate ligands and to characterize potential inhibitors found in milk. Milk from women with the secretor phenotype was strongly inhibitory, unlike milk from women that are non-secretors, which is devoid of 2-linked fucosylated structures. At least two fractions in human milk acted as inhibitors for the NV capsid attachment. The first fraction corresponded to BSSL (bile-salt-stimulated lipase) and the second to associated mucins MUC1 and MUC4. These proteins present tandem repeat O-glycosylated sequences that should act as decoy receptors for the NV, depending on the combined mother/child secretor status.
Figures



References
-
- Fankhauser R. L., Noel J. S., Monroe S. S., Ando T., Glass R. I. Molecular epidemiology of Norwalk-like viruses in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 1998;178:1571–1578. - PubMed
-
- Bon F., Fascia P., Dauvergne M., Tenenbaum D., Planson H., Petion A. M., Pothier P., Kohli E. Prevalence of group A rotavirus, human calicivirus, astrovirus and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J. Clin. Microbiol. 1999;37:3055–3058. - PMC - PubMed
-
- Pang X.-L., Honma S., Nakata S., Vesikari T. Human caliciviruses in acute gastroenteritis of young children in the community. J. Infect. Dis. 2000;81:S288–S294. - PubMed
-
- Parashar U. D., Li J. F., Cama R., DeZalia M., Monroe S. S., Taylor D. N., Figueroa D., Gilman R. H., Glass R. I. Human caliciviruses as a cause of severe gastroenteritis in peruvian children. J. Infect. Dis. 2004;190:1088–1092. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

