Dietary HDAC inhibitors: time to rethink weak ligands in cancer chemoprevention?
- PMID: 16267097
- PMCID: PMC2267878
- DOI: 10.1093/carcin/bgi253
Dietary HDAC inhibitors: time to rethink weak ligands in cancer chemoprevention?
Abstract
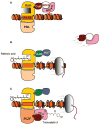
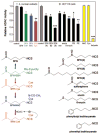
There is growing interest in the various mechanisms that regulate chromatin remodeling, including modulation of histone deacetylase (HDAC) activities. Competitive HDAC inhibitors disrupt the cell cycle and/or induce apoptosis via de-repression of genes such as P21 and BAX, and cancer cells appear to be more sensitive than non-transformed cells to trichostatin A and related HDAC inhibitory compounds. This apparent selectivity of action in cancer cells makes HDAC inhibitors an attractive avenue for drug development. However, in the search for potent HDAC inhibitors with cancer therapeutic potential there has been a tendency to overlook or dismiss weak ligands that could prove effective in cancer prevention, including agents present in the human diet. Recent reports have described butyrate, diallyl disulfide and sulforaphane as HDAC inhibitors, and many other dietary agents will be probably discovered to attenuate HDAC activity. Here we discuss 'pharmacologic' agents that potently de-repress gene expression (e.g. during therapeutic intervention) versus dietary HDAC inhibitors that, as weak ligands, might subtly regulate the expression of genes involved in cell growth and apoptosis. An important question is the extent to which dietary HDAC inhibitors, and other dietary agents that affect gene expression via chromatin remodeling, modulate the expression of genes such as P21 and BAX so that cells can respond most effectively to external stimuli and toxic insults.
Conflict of interest statement
Figures

Similar articles
-
Dietary agents as histone deacetylase inhibitors.Mol Carcinog. 2006 Jun;45(6):443-6. doi: 10.1002/mc.20224. Mol Carcinog. 2006. PMID: 16652377 Free PMC article. Review.
-
Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane.Curr Drug Targets. 2006 Apr;7(4):443-52. doi: 10.2174/138945006776359467. Curr Drug Targets. 2006. PMID: 16611031 Review.
-
Plant Isoquinoline Alkaloid Berberine Exhibits Chromatin Remodeling by Modulation of Histone Deacetylase To Induce Growth Arrest and Apoptosis in the A549 Cell Line.J Agric Food Chem. 2016 Dec 21;64(50):9542-9550. doi: 10.1021/acs.jafc.6b04453. Epub 2016 Dec 12. J Agric Food Chem. 2016. PMID: 27936791
-
Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention.J Nutr. 2009 Dec;139(12):2393-6. doi: 10.3945/jn.109.113332. Epub 2009 Oct 7. J Nutr. 2009. PMID: 19812222 Free PMC article.
-
Dietary histone deacetylase inhibitors: from cells to mice to man.Semin Cancer Biol. 2007 Oct;17(5):363-9. doi: 10.1016/j.semcancer.2007.04.001. Epub 2007 May 5. Semin Cancer Biol. 2007. PMID: 17555985 Free PMC article. Review.
Cited by
-
Targeting the epigenome with bioactive food components for cancer prevention.J Nutrigenet Nutrigenomics. 2011;4(5):275-92. doi: 10.1159/000334585. Epub 2012 Feb 22. J Nutrigenet Nutrigenomics. 2011. PMID: 22353664 Free PMC article. Review.
-
Resveratrol in prevention and treatment of common clinical conditions of aging.Clin Interv Aging. 2008;3(2):331-9. Clin Interv Aging. 2008. PMID: 18686754 Free PMC article. Review.
-
The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon.Cell Metab. 2011 May 4;13(5):517-26. doi: 10.1016/j.cmet.2011.02.018. Cell Metab. 2011. PMID: 21531334 Free PMC article.
-
Functional annotation of the cattle genome through systematic discovery and characterization of chromatin states and butyrate-induced variations.BMC Biol. 2019 Aug 16;17(1):68. doi: 10.1186/s12915-019-0687-8. BMC Biol. 2019. PMID: 31419979 Free PMC article.
-
Tocotrienols Attenuate White Adipose Tissue Accumulation and Improve Serum Cholesterol Concentration in High-Fat Diet-Treated Mice.Molecules. 2022 Mar 28;27(7):2188. doi: 10.3390/molecules27072188. Molecules. 2022. PMID: 35408585 Free PMC article.
References
-
- Szyf M. DNA methylation and demethylation as targets for anticancer therapy. Biochemistry (Mosc) 2005;70:533–549. - PubMed
-
- Cosgrove MS, Wolberger C. How does the histone code work? Biochem Cell Biol. 2005;83:468–476. - PubMed
-
- Fraga MF, Ballestar E, Villar-Garea A, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37:391–400. - PubMed
-
- Allison SJ, Milner J. Remodelling chromatin on a global scale: a novel protective function of p53. Carcinogenesis. 2004;25:1551–1557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

