Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae
- PMID: 16267135
- PMCID: PMC1283802
- DOI: 10.1073/pnas.0505350102
Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae
Abstract
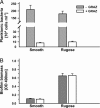
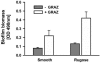
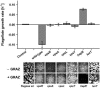
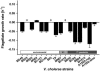
Persistence of the opportunistic bacterial pathogen Vibrio cholerae in aquatic environments is the principal cause for seasonal occurrence of cholera epidemics. This causality has been explained by postulating that V. cholerae forms biofilms in association with animate and inanimate surfaces. Alternatively, it has been proposed that bacterial pathogens are an integral part of the natural microbial food web and thus their survival is constrained by protozoan predation. Here, we report that both explanations are interrelated. Our data show that biofilms are the protective agent enabling V. cholerae to survive protozoan grazing while their planktonic counterparts are eliminated. Grazing on planktonic V. cholerae was found to select for the biofilm-enhancing rugose phase variant, which is adapted to the surface-associated niche by the production of exopolymers. Interestingly, grazing resistance in V. cholerae biofilms was not attained by exopolymer production alone but was accomplished by the secretion of an antiprotozoal factor that inhibits protozoan feeding activity. We identified that the cell density-dependent regulator hapR controls the production of this factor in biofilms. The inhibitory effect of V. cholerae biofilms was found to be widespread among toxigenic and nontoxigenic isolates. Our results provide a mechanistic explanation for the adaptive advantage of surface-associated growth in the environmental persistence of V. cholerae and suggest an important contribution of protozoan predation in the selective enrichment of biofilm-forming strains in the out-of-host environment.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

