Neuronal L-type calcium channels open quickly and are inhibited slowly
- PMID: 16267232
- PMCID: PMC6725800
- DOI: 10.1523/JNEUROSCI.1089-05.2005
Neuronal L-type calcium channels open quickly and are inhibited slowly
Abstract
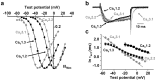
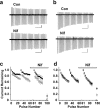
Neuronal L-type calcium channels are essential for regulating activity-dependent gene expression, but they are thought to open too slowly to contribute to action potential-dependent calcium entry. A complication of studying native L-type channels is that they represent a minor fraction of the whole-cell calcium current in most neurons. Dihydropyridine antagonists are therefore widely used to establish the contribution of L-type channels to various neuronal processes and to study their underlying biophysical properties. The effectiveness of these antagonists on L-type channels, however, varies with stimulus and channel subtype. Here, we study recombinant neuronal L-type calcium channels, CaV1.2 and CaV1.3. We show that these channels open with fast kinetics and carry substantial calcium entry in response to individual action potential waveforms, contrary to most studies of native L-type currents. Neuronal CaV1.3 L-type channels were as efficient as CaV2.2 N-type channels at supporting calcium entry during action potential-like stimuli. We conclude that the apparent slow activation of native L-type currents and their lack of contribution to single action potentials reflect the state-dependent nature of the dihydropyridine antagonists used to study them, not the underlying properties of L-type channels.
Figures


References
-
- Birnbaumer L, Qin N, Olcese R, Tareilus E, Platano D, Costantin J, Stefani E (1998) Structures and functions of calcium channel beta subunits. J Bioenerg Biomembr 30: 357-375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
