Molecular interaction maps of bioregulatory networks: a general rubric for systems biology
- PMID: 16267266
- PMCID: PMC1345641
- DOI: 10.1091/mbc.e05-09-0824
Molecular interaction maps of bioregulatory networks: a general rubric for systems biology
Abstract
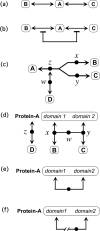
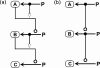
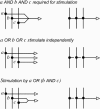
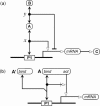
A standard for bioregulatory network diagrams is urgently needed in the same way that circuit diagrams are needed in electronics. Several graphical notations have been proposed, but none has become standard. We have prepared many detailed bioregulatory network diagrams using the molecular interaction map (MIM) notation, and we now feel confident that it is suitable as a standard. Here, we describe the MIM notation formally and discuss its merits relative to alternative proposals. We show by simple examples how to denote all of the molecular interactions commonly found in bioregulatory networks. There are two forms of MIM diagrams. "Heuristic" MIMs present the repertoire of interactions possible for molecules that are colocalized in time and place. "Explicit" MIMs define particular models (derived from heuristic MIMs) for computer simulation. We show also how pathways or processes can be highlighted on a canonical heuristic MIM. Drawing a MIM diagram, adhering to the rules of notation, imposes a logical discipline that sharpens one's understanding of the structure and function of a network.
Figures








References
-
- Aladjem, M. I., Pasa, S., Parodi, S., Weinstein, J. N., Pommier, Y., and Kohn, K. W. (2004). Molecular interaction maps–a diagrammatic graphical language for bioregulatory networks. Sci STKE 2004, pe8. - PubMed
-
- Corney, D. P., Buxton, B. F., Langdon, W. B., and Jones, D. T. (2004). BioRAT: extracting biological information from full-length papers. Bioinformatics 20, 3206 –3213. - PubMed
-
- Funahashi, A., Morohashi, M., and Kitano, H. (2003). CellDesigner: a process diagram editor for gene-regulatory and biochemical networks. Biosilico 1, 159 –162.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

