Catabolite repression and activation in Bacillus subtilis: dependency on CcpA, HPr, and HprK
- PMID: 16267306
- PMCID: PMC1280314
- DOI: 10.1128/JB.187.22.7826-7839.2005
Catabolite repression and activation in Bacillus subtilis: dependency on CcpA, HPr, and HprK
Abstract
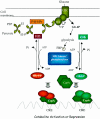
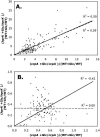
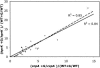
Previous studies have suggested that the transcription factor CcpA, as well as the coeffectors HPr and Crh, both phosphorylated by the HprK kinase/phosphorylase, are primary mediators of catabolite repression and catabolite activation in Bacillus subtilis. We here report whole transcriptome analyses that characterize glucose-dependent gene expression in wild-type cells and in isogenic mutants lacking CcpA, HprK, or the HprK phosphorylatable serine in HPr. Binding site identification revealed which genes are likely to be primarily or secondarily regulated by CcpA. Most genes subject to CcpA-dependent regulation are regulated fully by HprK and partially by serine-phosphorylated HPr [HPr(Ser-P)]. A positive linear correlation was noted between the dependencies of catabolite-repressible gene expression on CcpA and HprK, but no such relationship was observed for catabolite-activated genes, suggesting that large numbers of the latter genes are not regulated by the CcpA-HPr(Ser-P) complex. Many genes that mediate nitrogen or phosphorus metabolism as well as those that function in stress responses proved to be subject to CcpA-dependent glucose control. While nitrogen-metabolic genes may be subject to either glucose repression or activation, depending on the gene, almost all glucose-responsive phosphorus-metabolic genes exhibit activation while almost all glucose-responsive stress genes show repression. These responses are discussed from physiological standpoints. These studies expand our appreciation of CcpA-mediated catabolite control and provide insight into potential interregulon control mechanisms in gram-positive bacteria.
Figures
Similar articles
-
How seryl-phosphorylated HPr inhibits PrfA, a transcription activator of Listeria monocytogenes virulence genes.J Mol Microbiol Biotechnol. 2005;9(3-4):224-34. doi: 10.1159/000089650. J Mol Microbiol Biotechnol. 2005. PMID: 16415595
-
Malate-mediated carbon catabolite repression in Bacillus subtilis involves the HPrK/CcpA pathway.J Bacteriol. 2011 Dec;193(24):6939-49. doi: 10.1128/JB.06197-11. Epub 2011 Oct 14. J Bacteriol. 2011. PMID: 22001508 Free PMC article.
-
Antitermination by GlpP, catabolite repression via CcpA and inducer exclusion triggered by P-GlpK dephosphorylation control Bacillus subtilis glpFK expression.Mol Microbiol. 2002 Feb;43(4):1039-52. doi: 10.1046/j.1365-2958.2002.02800.x. Mol Microbiol. 2002. PMID: 11929549
-
HPr kinase/phosphorylase, a Walker motif A-containing bifunctional sensor enzyme controlling catabolite repression in Gram-positive bacteria.Biochim Biophys Acta. 2004 Mar 11;1697(1-2):123-35. doi: 10.1016/j.bbapap.2003.11.018. Biochim Biophys Acta. 2004. PMID: 15023355 Review.
-
Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the gram-positive bacteria?Mol Microbiol. 1995 Feb;15(3):395-401. doi: 10.1111/j.1365-2958.1995.tb02252.x. Mol Microbiol. 1995. PMID: 7540244 Review.
Cited by
-
Bacillus cereus Decreases NHE and CLO Exotoxin Synthesis to Maintain Appropriate Proteome Dynamics During Growth at Low Temperature.Toxins (Basel). 2020 Oct 6;12(10):645. doi: 10.3390/toxins12100645. Toxins (Basel). 2020. PMID: 33036317 Free PMC article.
-
GapB Is Involved in Biofilm Formation Dependent on LrgAB but Not the SinI/R System in Bacillus cereus 0-9.Front Microbiol. 2020 Dec 7;11:591926. doi: 10.3389/fmicb.2020.591926. eCollection 2020. Front Microbiol. 2020. PMID: 33365021 Free PMC article.
-
A native conjugative plasmid confers potential selective advantages to plant growth-promoting Bacillus velezensis strain GH1-13.Commun Biol. 2021 May 14;4(1):582. doi: 10.1038/s42003-021-02107-z. Commun Biol. 2021. PMID: 33990691 Free PMC article.
-
Effects of oxygen on virulence traits of Streptococcus mutans.J Bacteriol. 2007 Dec;189(23):8519-27. doi: 10.1128/JB.01180-07. Epub 2007 Oct 5. J Bacteriol. 2007. PMID: 17921307 Free PMC article.
-
ManLMN is a glucose transporter and central metabolic regulator in Streptococcus pneumoniae.Mol Microbiol. 2016 Nov;102(3):467-487. doi: 10.1111/mmi.13473. Epub 2016 Aug 18. Mol Microbiol. 2016. PMID: 27472033 Free PMC article.
References
-
- Adhya, S. 1987. The galactose operon, p. 1503-1512. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella typhimurium:: Cellular and molecular biology, vol. 2. ASM Press, Washington, D.C.
-
- Aung-Hilbrich, L. M., G. Seidel, A. Wagner, and W. Hillen. 2002. Quantification of the influence of HPr-Ser46-P on CcpA-cre interaction. J. Mol. Biol. 319:77-85. - PubMed
-
- Blencke, H. M., G. Homuth, H. Ludwig, U. Mader, M. Hecker, and J. Stülke. 2003. Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab. Eng. 5:133-149. - PubMed
-
- Böel, G., I. Mijakovic, A. Maze, S. Poncet, M. K. Taha, M. Larribe, E. Darbon, A. Khemiri, A. Galinier, and J. Deutscher. 2003. Transcription regulators potentially controlled by HPr kinase/phosphorylase in Gram-negative bacteria. J. Mol. Microbiol. Biotechnol. 5:206-215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

