Galphaq binds to p110alpha/p85alpha phosphoinositide 3-kinase and displaces Ras
- PMID: 16268778
- PMCID: PMC1383705
- DOI: 10.1042/BJ20051493
Galphaq binds to p110alpha/p85alpha phosphoinositide 3-kinase and displaces Ras
Abstract
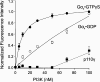
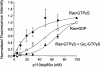
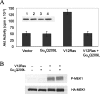
Several studies have reported that activation of G(q)-coupled receptors inhibits PI3K (phosphoinositide 3-kinase) signalling. In the present study, we used purified proteins to demonstrate that Galpha(q) directly inhibits p110alpha/p85alpha PI3K in a GTP-dependent manner. Activated Galpha(q) binds to the p110alpha/p85alpha PI3K with an apparent affinity that is seven times stronger than that for Galpha(q).GDP as measured by fluorescence spectroscopy. In contrast, Galpha(q) did not bind to the p110gamma PI3K. Fluorescence spectroscopy experiments also showed that Galpha(q) competes with Ras, a PI3K activator, for binding to p110alpha/p85alpha. Interestingly, co-precipitation studies using deletion mutants showed that Galpha(q) binds to the p85-binding domain of p110alpha and not to the Ras-binding domain. Expression of constitutively active Galpha(q)Q209L in cells inhibited Ras activation of the PI3K/Akt pathway but had no effect on Ras/Raf/MEK [MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase] signalling. These results suggest that activation of G(q)-coupled receptors leads to increased binding of Galpha(q).GTP to some isoforms of PI3K, which might explain why these receptors inhibit this signalling pathway in certain cell types.
Figures
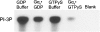

Similar articles
-
Activated G alpha q inhibits p110 alpha phosphatidylinositol 3-kinase and Akt.J Biol Chem. 2003 Jun 27;278(26):23472-9. doi: 10.1074/jbc.M212232200. Epub 2003 Apr 18. J Biol Chem. 2003. PMID: 12704201
-
Activation of Ras-dependent signaling pathways by G(14) -coupled receptors requires the adaptor protein TPR1.J Cell Biochem. 2012 Nov;113(11):3486-97. doi: 10.1002/jcb.24225. J Cell Biochem. 2012. PMID: 22711498
-
Galpha16 interacts with Class IA phosphatidylinositol 3-kinases and inhibits Akt signaling.Cell Signal. 2010 Sep;22(9):1379-87. doi: 10.1016/j.cellsig.2010.05.008. Epub 2010 May 12. Cell Signal. 2010. PMID: 20471473
-
Gαq signalling: the new and the old.Cell Signal. 2014 May;26(5):833-48. doi: 10.1016/j.cellsig.2014.01.010. Epub 2014 Jan 17. Cell Signal. 2014. PMID: 24440667 Review.
-
Class I phosphoinositide 3-kinase (PI3K) regulatory subunits and their roles in signaling and disease.Adv Biol Regul. 2020 Jan;75:100657. doi: 10.1016/j.jbior.2019.100657. Epub 2019 Sep 28. Adv Biol Regul. 2020. PMID: 31611073 Review.
Cited by
-
The phosphoinositide 3-kinase α selective inhibitor BYL719 enhances the effect of the protein kinase C inhibitor AEB071 in GNAQ/GNA11-mutant uveal melanoma cells.Mol Cancer Ther. 2014 May;13(5):1044-53. doi: 10.1158/1535-7163.MCT-13-0550. Epub 2014 Feb 21. Mol Cancer Ther. 2014. PMID: 24563540 Free PMC article.
-
Mechanistic Insights into the Anticancer Potential of Methoxyflavones Analogs: A Review.Molecules. 2025 Jan 16;30(2):346. doi: 10.3390/molecules30020346. Molecules. 2025. PMID: 39860214 Free PMC article. Review.
-
Galphaq binds two effectors separately in cells: evidence for predetermined signaling pathways.Biophys J. 2008 Sep;95(5):2575-82. doi: 10.1529/biophysj.108.129353. Epub 2008 May 30. Biophys J. 2008. PMID: 18515384 Free PMC article.
-
Gq-coupled purinergic receptors inhibit insulin-like growth factor-I/phosphoinositide 3-kinase pathway-dependent keratinocyte migration.Mol Biol Cell. 2010 Mar 15;21(6):946-55. doi: 10.1091/mbc.e09-06-0497. Epub 2010 Jan 20. Mol Biol Cell. 2010. PMID: 20089844 Free PMC article.
-
Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Gα/q,11.Front Cardiovasc Med. 2015 Mar 24;2:14. doi: 10.3389/fcvm.2015.00014. eCollection 2015. Front Cardiovasc Med. 2015. PMID: 26664886 Free PMC article. Review.
References
-
- Vanhaesebroeck B., Leevers S. J., Ahmadi K., Timms J., Katso R., Driscoll P. C., Woscholski R., Parker P. J., Waterfield M. D. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001;70:535–602. - PubMed
-
- Oudit G. Y., Sun H., Kerfant B. G., Crackower M. A., Penninger J. M., Backx P. H. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J. Mol. Cell. Cardiol. 2004;37:449–471. - PubMed
-
- Suire S., Coadwell J., Ferguson G. J., Davidson K., Hawkins P., Stephens L. p84, a new Gbetagamma-activated regulatory subunit of the type IB phosphoinositide 3-kinase p110gamma. Curr. Biol. 2005;15:566–570. - PubMed
-
- Kodaki T., Woscholski R., Hallberg B., Rodriguez-Viciana P., Downward J., Parker P. J. The activation of phosphatidylinositol 3-kinase by Ras. Curr. Biol. 1994;4:798–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

