Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis
- PMID: 16270031
- PMCID: PMC1283956
- DOI: 10.1038/sj.emboj.7600857
Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis
Abstract
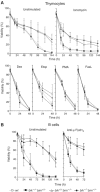
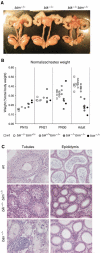
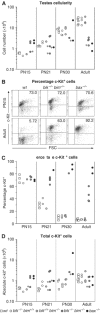
The BH3-only proteins of the Bcl-2 family initiate apoptosis through the activation of Bax-like relatives. Loss of individual BH3-only proteins can lead either to no phenotype, as in mice lacking Bik, or to marked cell excess, as in the hematopoietic compartment of animals lacking Bim. To investigate whether functional redundancy with Bim might obscure a significant role for Bik, we generated mice lacking both genes. The hematopoietic compartments of bik-/-bim-/- and bim-/- mice were indistinguishable. However, although testes develop normally in mice lacking either Bik or Bim, adult bik-/-bim-/- males were infertile, with reduced testicular cellularity and no spermatozoa. The testis of young bik-/-bim-/- males, like those lacking Bax, exhibited increased numbers of spermatogonia and spermatocytes, although loss of Bik plus Bim blocked spermatogenesis somewhat later than Bax deficiency. The initial excess of early germ cells suggests that spermatogenesis fails because supporting Sertoli cells are overwhelmed. Thus, Bik and Bim share, upstream of Bax, the role of eliminating supernumerary germ cells during the first wave of spermatogenesis, a process vital for normal testicular development.
Figures





References
-
- Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev 17: 2481–2495 - PubMed
-
- Akiyama T, Bouillet P, Miyazaki T, Kadono Y, Chikuda H, Chung UI, Fukuda A, Hikita A, Seto H, Okada T, Inaba T, Sanjay A, Baron R, Kawaguchi H, Oda H, Nakamura K, Strasser A, Tanaka S (2003) Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J 22: 6653–6664 - PMC - PubMed
-
- Allan DJ, Harmon BV, Kerr JFR (1987) Cell death in spermatogenesis. In Perspectives on Mammalian Cell Death, Potten CS (ed) pp 229–258. Oxford: Oxford University Press
-
- Bouillet P, Cory S, Zhang L-C, Strasser A, Adams JM (2001) Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev Cell 1: 645–653 - PubMed
-
- Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F, Adams JM, Strasser A (1999) Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286: 1735–1738 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

