PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility
- PMID: 16270034
- PMCID: PMC1283946
- DOI: 10.1038/sj.emboj.7600847
PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility
Abstract
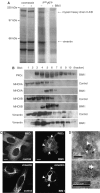
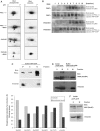
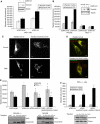
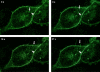
PKCepsilon controls the transport of endocytosed beta1-integrins to the plasma membrane regulating directional cell motility. Vimentin, an intermediate filament protein upregulated upon epithelial cell transformation, is shown here to be a proximal PKCepsilon target within the recycling integrin compartment. On inhibition of PKC and vimentin phosphorylation, integrins become trapped in vesicles and directional cell motility towards matrix is severely attenuated. In vitro reconstitution assays showed that PKCepsilon dissociates from integrin containing endocytic vesicles in a selectively phosphorylated vimentin containing complex. Mutagenesis of PKC (controlled) sites on vimentin and ectopic expression of the variant leads to the accumulation of intracellular PKCepsilon/integrin positive vesicles. Finally, introduction of ectopic wild-type vimentin is shown to promote cell motility in a PKCepsilon-dependent manner; alanine substitutions in PKC (controlled) sites on vimentin abolishes the ability of vimentin to induce cell migration, whereas the substitution of these sites with acidic residues enables vimentin to rescue motility of PKCepsilon null cells. Our results indicate that PKC-mediated phosphorylation of vimentin is a key process in integrin traffic through the cell.
Figures

References
-
- Bretscher MS (1996) Moving membrane up to the front of migrating cells. Cell 85: 465–467 - PubMed
-
- Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P (2000) Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci 113 (Part 13): 2455–2462 - PubMed
-
- Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, Langa F, Aumailley M, Delouvee A, Koteliansky V, Babinet C, Krieg T (1998) Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci 111 (Part 13): 1897–1907 - PubMed
-
- Eriksson JE, He T, Trejo-Skalli AV, Harmala-Brasken AS, Hellman J, Chou YH, Goldman RD (2004) Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci 117: 919–932 - PubMed
-
- Franke WW, Hergt M, Grund C (1987) Rearrangement of the vimentin cytoskeleton during adipose conversion: formation of an intermediate filament cage around lipid globules. Cell 49: 131–141 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

