Revealing the large-scale network organization of growth hormone-secreting cells
- PMID: 16272219
- PMCID: PMC1277257
- DOI: 10.1073/pnas.0508202102
Revealing the large-scale network organization of growth hormone-secreting cells
Abstract
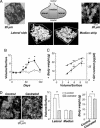
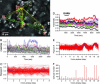
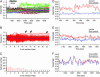
Pituitary growth hormone (GH)-secreting cells regulate growth and metabolism in animals and humans. To secrete highly ordered GH pulses (up to 1,000-fold rise in hormone levels in vivo), the pituitary GH cell population needs to mount coordinated responses to GH secretagogues, yet GH cells display an apparently heterogeneous scattered distribution in 2D histological studies. To address this paradox, we analyzed in 3D both positioning and signaling of GH cells using reconstructive, two-photon excitation microscopy to image the entire pituitary in GH-EGFP transgenic mice. Our results unveiled a homologous continuum of GH cells connected by adherens junctions that wired the whole gland and exhibited the three primary features of biological networks: robustness of architecture across lifespan, modularity correlated with pituitary GH contents and body growth, and connectivity with spatially stereotyped motifs of cell synchronization coordinating cell activity. These findings change our view of GH cells, from a collection of dispersed cells to a geometrically connected homotypic network of cells whose local morphology and connectivity can vary, to alter the timing of cellular responses to promote more coordinated pulsatile secretion. This large-scale 3D view of cell functioning provides a powerful approach to identify and understand other networks of endocrine cells that are thought to be scattered in situ. Many dispersed endocrine systems exhibit pulsatile outputs. We suggest that cell positioning and associated cell-cell connection mechanisms will be critical parameters that determine how well such systems can deliver a coordinated secretory pulse of hormone to their target tissues.
Figures


References
-
- Kostyo, J. L. & Goodman, H. M. (1999) Hormonal Control of Growth (Oxford Univ. Press, Oxford).
-
- Asa, S. L. & Ezzat, S. (2002) Nat. Rev. Cancer 2, 836-849. - PubMed
-
- Bowers, C. Y., Reynolds, G. A., Durham, D., Barrera, C. M., Pezzoli, S. S. & Thorner, M. O. (1990) J. Clin. Endocrinol. Metab. 70, 975-982. - PubMed
-
- Clarke, R. G. & Robinson, I. C. A. F. (1985) Nature 106, 281-289.
-
- Tannenbaum, G. S. & Martin, J. B. (1976) Endocrinology 98, 562-570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

